The New England Journal of Medicine dessa semana em artigo com acesso direto no site http://www.nejm.org apresenta a situação dos médicos de Gana, que tal os jogadores de futebol, estão abandonando seu país para atuar no Reino Unido e Estados Unidos. Essa situação está piorando a assistência naquele país. Porém, Cuba, sim de Cuba já vieram 200 médicos para o país africano. Ou seja, saem os melhores para Estados Unidos e Europa e, vêem os cubanos. Interessante essa nova forma de divisão mundial do trabalho médico.
quarta-feira, 31 de janeiro de 2007
terça-feira, 30 de janeiro de 2007
Médicos cubanos na Venezuela: seu nome é escravidão.
No Estadão (27/01/07), Paulo Moreira Leite fez uma excelente descrição do médico cubano na Venezuela no link abaixo. A família do médico permanece em Cuba, sua ativididade é controlada, não há liberdade de ir e vir....o que parece isso? liberdade profissional? ou escravidão ou semi-escravidão? Hoje, publica em seu blog o seguinte comentário:
Médicos cubanos, venezuelanos e brasileiros
por Paulo Moreira LeiteAuthor Seção: brasil às 09:56:25.
Conversei com um antigo ministro da Saúde sobre o balanço da OMS na Venzuela, que mostra um progresso importante no primeiro atendimento nos bairros pobres depois que o governo de Hugo Chávez assinou um convênio com Fidel Castro, onde troca petróleo por médicos e enfermeiros. Ele admitiu: “não é novidade.” A origem do problema, como se sabe, é que os médicos venezuelanos não querem trabalhar nos bairros pobres. “Esse problema também ocorre no Brasil. Nem adianta subsidiar, oferecer ajuda para o transporte e um salário mais alto. A maioria não se interessa. Sem falar naqueles que aceitam o emprego, pegam o salário mas não aparecem no local de trabalho.”Anos atrás, o governo brasileiro chegou a cogitar uma cooperação com o regime cubano, importando médicos formados na Ilha. A idéia não foi adiante por diversas razões – inclusive pelo receio de se produzir um escândalo político. Mas desde então Brasília faz vista grossa para prefeituras e governos de Estado que mantém convênios desse tipo com o regime de Fidel Castro.Leia mais sobre isso emhttp://txt.estado.com.br/editorias/2007/01/27/int-1.93.9.20070127.7.1.xml
por Paulo Moreira LeiteAuthor Seção: brasil às 09:56:25.
Conversei com um antigo ministro da Saúde sobre o balanço da OMS na Venzuela, que mostra um progresso importante no primeiro atendimento nos bairros pobres depois que o governo de Hugo Chávez assinou um convênio com Fidel Castro, onde troca petróleo por médicos e enfermeiros. Ele admitiu: “não é novidade.” A origem do problema, como se sabe, é que os médicos venezuelanos não querem trabalhar nos bairros pobres. “Esse problema também ocorre no Brasil. Nem adianta subsidiar, oferecer ajuda para o transporte e um salário mais alto. A maioria não se interessa. Sem falar naqueles que aceitam o emprego, pegam o salário mas não aparecem no local de trabalho.”Anos atrás, o governo brasileiro chegou a cogitar uma cooperação com o regime cubano, importando médicos formados na Ilha. A idéia não foi adiante por diversas razões – inclusive pelo receio de se produzir um escândalo político. Mas desde então Brasília faz vista grossa para prefeituras e governos de Estado que mantém convênios desse tipo com o regime de Fidel Castro.Leia mais sobre isso emhttp://txt.estado.com.br/editorias/2007/01/27/int-1.93.9.20070127.7.1.xml
Ou seja, sobrou para o médico brasileiro, o comentário de ministro não-identificado cujo conteúdo merece contestação. Mas, fica para outro momento.
PLOS Medicine: imperdível.
Imperdível, a mais recente edição de PLOS Medicine, a Public Library of Medicine.
Acesso livre, obviamente.
em
http://medicine.plosjournals.org
Vejam os principais artigos:
Does Industry Sponsorship Undermine the Integrity of Nutrition Research?
Acesso livre, obviamente.
em
http://medicine.plosjournals.org
Vejam os principais artigos:
Does Industry Sponsorship Undermine the Integrity of Nutrition Research?
Authors, Ghosts, Damned Lies, and Statisticians
Measles Still Has a Devastating Impact in Unvaccinated Populations
When Is Replacement Feeding Safe for Infants of HIV-Infected Women?
Worldmapper: The Human Anatomy of a Small Planet
XDR-TB in South Africa: No Time for Denial or Complacency
The Structure and Function of Research Ethics Committees in Africa: A Case Study
Epidemiology of Tuberculosis in a High HIV Prevalence Population Provided with Enhanced Diagnosis of Symptomatic DiseaseTobacco Smoke, Indoor Air Pollution and Tuberculosis: A Systematic Review and Meta-Analysis
Measles Still Has a Devastating Impact in Unvaccinated Populations
When Is Replacement Feeding Safe for Infants of HIV-Infected Women?
Worldmapper: The Human Anatomy of a Small Planet
XDR-TB in South Africa: No Time for Denial or Complacency
The Structure and Function of Research Ethics Committees in Africa: A Case Study
Epidemiology of Tuberculosis in a High HIV Prevalence Population Provided with Enhanced Diagnosis of Symptomatic DiseaseTobacco Smoke, Indoor Air Pollution and Tuberculosis: A Systematic Review and Meta-Analysis
segunda-feira, 29 de janeiro de 2007
Tailândia suspende patentes do Plavix e Kaletra.
Ditaduras são uma "beleza para a saúde pública". Basta um decreto e, pronto, encerra-se a questão. Bem, estamos na democracia com poderes legislativo e judiciário e, ministério público, imprensa livre e com convenções internacionais em vigor. A Tailândia suspendeu a patente do Plavix (pós-infarto do miocárdio e acidente vascular cerebral) e do Kaletra (HIV). O Brasil é citado como país que bateu firme e, conseguiu redução do preço do Kaletra. Detalhe para influência da indústria farmacêutica indiana nessa decisão. Abaixo, trechos da reportagem do The Wall Street Journal.
Thailand Suspends Patents on Two Drugs
By NICHOLAS ZAMISKAJanuary 29, 2007 1:48 p.m.
HONG KONG -- A decision by Thailand's new, military-installed government to increase access to drugs by suspending patent protections on a heart-disease treatment and an HIV medication highlights a growing tension over intellectual-property rights versus public-health interests.
The decision was criticized by the pharmaceutical industry, which said Bangkok is considering allowing copycat versions of more drugs in the near future. The government's move was notable partly because it included the heart medicine, expanding the realm of drugs over which such conflicts have typically occurred. Thailand's Ministry of Health confirmed Monday that the government had approved the sale and production of cheap, generic versions of Plavix, the blood-thinning drug originally developed by Sanofi-Aventis SA, of Paris, and now co-marketed in several countries with Bristol-Myers Squibb Co., of New York, and the HIV treatment Kaletra, made by Abbott Laboratories, of the U.S. World Trade Organization rules allow a government to unilaterally declare an emergency and make or sell patented drugs without the permission of the drug companies.
By NICHOLAS ZAMISKAJanuary 29, 2007 1:48 p.m.
HONG KONG -- A decision by Thailand's new, military-installed government to increase access to drugs by suspending patent protections on a heart-disease treatment and an HIV medication highlights a growing tension over intellectual-property rights versus public-health interests.
The decision was criticized by the pharmaceutical industry, which said Bangkok is considering allowing copycat versions of more drugs in the near future. The government's move was notable partly because it included the heart medicine, expanding the realm of drugs over which such conflicts have typically occurred. Thailand's Ministry of Health confirmed Monday that the government had approved the sale and production of cheap, generic versions of Plavix, the blood-thinning drug originally developed by Sanofi-Aventis SA, of Paris, and now co-marketed in several countries with Bristol-Myers Squibb Co., of New York, and the HIV treatment Kaletra, made by Abbott Laboratories, of the U.S. World Trade Organization rules allow a government to unilaterally declare an emergency and make or sell patented drugs without the permission of the drug companies.
In fact, a government's ability to suspend a company's drug patent can prove a powerful bargaining chip in reducing prices. In July 2005, for instance, Brazil reached an agreement with Abbott that lowered the price of Kaletra while preserving the company's patent on the drug.
Indian generics makers, which for years have benefited from that country's lax patent laws, stand to benefit from the Thai decision. Bangkok is considering making its purchases from Hetero Drugs Ltd. and Cipla Ltd., both of India, according to Thawat Suntrajarn, director general of the Ministry of Health's department of disease control.
Indian generics makers, which for years have benefited from that country's lax patent laws, stand to benefit from the Thai decision. Bangkok is considering making its purchases from Hetero Drugs Ltd. and Cipla Ltd., both of India, according to Thawat Suntrajarn, director general of the Ministry of Health's department of disease control.
Dr. Thawat said the government's move will cut the price of the HIV drug Kaletra in half, reducing the monthly cost per patient to about 3,000 Thai baht ($89.55) or less. At current prices, the government can afford to provide medicine to only one-fifth of the 500,000 people living with the HIV virus in Thailand, Public Health Minister Mongkol Na Songkhla told the Associated Press, adding that the ministry was willing to talk to the companies about importing their drugs at cheaper prices.
domingo, 28 de janeiro de 2007
Exame de Ordem: uma opinião de Jânio de Freitas
Aos opositores do exame do CREMESP, uma advertência vinda de Jânio de Freitas, da Folha de S. Paulo.
JANIO DE FREITAS Doença brasileira
40% dos formandos em medicina não têm nem os conhecimentos mínimos para exercê-la
ENQUANTO se discutem os efeitos hipotéticos do pacote de um presidente que declarava encerrada, por seu governo, a era dos pacotes, uma notícia literalmente fatídica mal chegou à superfície: 40% dos formandos em medicina não têm nem os conhecimentos mínimos para exercê-la - mas logo estarão, ou já estão, atendendo como médicos. Não são formandos de faculdades em estados precários, Piauí, Amapá, Rondônia, mas de faculdades da riqueza paulista, submetidos ao veredicto do Conselho Regional de Medicina de São Paulo-Cremesp.Duas observações do coordenador do exame, cardiologista Bráulio Luna Filho, dimensionam a ênfase merecida, mas negada, pela notícia.Os formandos em geral iniciam a prática médica pela emergência hospitalar, onde a maioria dos pacientes chega necessitando de tratamento tão urgente quanto competente. Aí os aguarda a alta probabilidade de atendimento incompetente.A má formação manifesta-se com a mesma ameaça em outros setores, inclusive consultórios particulares, como se deduz das 3.360 denúncias de erros médicos em 2005, com aumento de 142% em apenas dez anos. Não por acaso, a maioria dos acusados foi formada, ou melhor, diplomada pelas faculdades com o desempenho mais deplorável no Exame Nacional de Desempenho dos Estudantes, o Enade.Do anterior para o recente exame aplicado pelo Cremesp, ficou evidente a piora constante do ensino de medicina, com mais 7% de reprovações só de um ano para o outro.E olhe que os formandos dispostos ao teste do Cremesp, não obrigatório, são tidos como os melhores de suas faculdades.Vê-se que o tal Enade não se prestou à utilidade tão propalada.Mais útil - aliás, só por absurdo ainda inexistente - é outro tipo de exame.Assim como a Ordem dos Advogados do Brasil exige a aprovação em exame seu, para dar validade profissional aos diplomas das faculdades de Direito, os diplomados em medicina não poderiam ser dispensados de exame pelas autoridades da profissão, para justificarem a confiança das vidas alheias.De garis, estes heróis das cidades, e de médicos depende a nossa existência de seres urbanos
40% dos formandos em medicina não têm nem os conhecimentos mínimos para exercê-la
ENQUANTO se discutem os efeitos hipotéticos do pacote de um presidente que declarava encerrada, por seu governo, a era dos pacotes, uma notícia literalmente fatídica mal chegou à superfície: 40% dos formandos em medicina não têm nem os conhecimentos mínimos para exercê-la - mas logo estarão, ou já estão, atendendo como médicos. Não são formandos de faculdades em estados precários, Piauí, Amapá, Rondônia, mas de faculdades da riqueza paulista, submetidos ao veredicto do Conselho Regional de Medicina de São Paulo-Cremesp.Duas observações do coordenador do exame, cardiologista Bráulio Luna Filho, dimensionam a ênfase merecida, mas negada, pela notícia.Os formandos em geral iniciam a prática médica pela emergência hospitalar, onde a maioria dos pacientes chega necessitando de tratamento tão urgente quanto competente. Aí os aguarda a alta probabilidade de atendimento incompetente.A má formação manifesta-se com a mesma ameaça em outros setores, inclusive consultórios particulares, como se deduz das 3.360 denúncias de erros médicos em 2005, com aumento de 142% em apenas dez anos. Não por acaso, a maioria dos acusados foi formada, ou melhor, diplomada pelas faculdades com o desempenho mais deplorável no Exame Nacional de Desempenho dos Estudantes, o Enade.Do anterior para o recente exame aplicado pelo Cremesp, ficou evidente a piora constante do ensino de medicina, com mais 7% de reprovações só de um ano para o outro.E olhe que os formandos dispostos ao teste do Cremesp, não obrigatório, são tidos como os melhores de suas faculdades.Vê-se que o tal Enade não se prestou à utilidade tão propalada.Mais útil - aliás, só por absurdo ainda inexistente - é outro tipo de exame.Assim como a Ordem dos Advogados do Brasil exige a aprovação em exame seu, para dar validade profissional aos diplomas das faculdades de Direito, os diplomados em medicina não poderiam ser dispensados de exame pelas autoridades da profissão, para justificarem a confiança das vidas alheias.De garis, estes heróis das cidades, e de médicos depende a nossa existência de seres urbanos
sábado, 27 de janeiro de 2007
Presidente decreta o fim da vitimização.
Sua Excia, o Presidente da República Federativa do Brasil na data de ontem proferiu frase histórica. Será o fim da vitimização coletiva?
“Nós temos que parar de viajar o mundo chorando a nossa miséria e importando culpados pela nossa desgraça. Muitas vezes, a responsabilidade é nossa”!
Tratamento da degeneração macular: marcha a ré importante.
Esse blog é sempre crítico quanto às novidades. No entanto, em 8/10/07 destacou a importância dos resultados do novo tratamento para da degeneração macular, uma causa cada vez mais importante de cegueira por que associada à idade. No entanto, salvei-me pelo parágrafo onde afirmei que:
Os primeiros ensaios clínicos foram “excitantes”. Vamos aguardar o que virá, porque se confirmar esses resultados a longo prazo com pouco risco haverá um tratamento efetivo para a doença.Ontem, a Genentech informou que o medicamento em questão aumentou a incidência de acidente vascular cerebral. Em outras palavras, para cada 110 tratamentos, um poderá provocar um caso de doença cerebrovascular. Várias revistas americanas e, uma cópia brasileira, afirmaram que esse foi o maior avanço da medicina em 2006. Infelizmente, não foi o caso. Abaixo a reportagem do The New York Times.
Eye Drug Might Raise Risk of Stroke, Genentech Says
By ANDREW POLLACK
Published: January 27, 2007
LOS ANGELES, Jan. 26 — Use of Genentech’s new eye drug, Lucentis, might raise the risk of stroke, the company said Friday.
The company posted on its Web site a letter it sent to retina specialists this week. According to the letter, 1.2 percent of patients treated with a high dose of Lucentis in a clinical trial suffered a stroke, compared with only 0.3 percent of those treated with a low dose. The difference was statistically significant.
The new findings could conceivably damp some demand for Lucentis, because the high dose, 0.5 milligrams per injection, is the one that is marketed. Shares of Genentech fell 89 cents, or 1 percent, to $86.57.
Dawn Kalmar, a spokeswoman for Genentech, said Friday that the company wanted to move quickly to communicate the information. But she said the company did not expect the new data to prompt a change in the drug’s label, which already contains a warning about risks of blood-clotting events like strokes.
Lucentis, also called ranibizumab, is approved to treat age-related macular degeneration, the leading cause of blindness in the elderly. It is the first drug shown in clinical trials to improve eyesight for a significant number of patients, as opposed to merely slowing the rate of vision loss.
Approved in June, the drug had sales of $380 million for the rest of 2006, an amount that greatly exceeded some analysts’ expectations. Genentech executives have cautioned, though, that sales growth may moderate as patients on the drug are treated less frequently.
The new data was preliminary, from 2,400 patients who had been followed for an average of 230 days since beginning treatment. The full study will continue and will follow them for a year.
Dr. Philip J. Rosenfeld, a retina specialist, said the preliminary data would not influence him because it could later change.
“Right now, it will have no impact on my use of Lucentis,” Dr. Rosenfeld, who is at the Bascom Palmer Eye Institute of the University of Miami, said in an e-mail message. “When given a choice between a high likelihood of blindness or a 1 percent risk of stroke, I think most patients will choose their vision.”
The label for Lucentis notes a theoretical risk of blood-clotting events like strokes and heart attacks, though it says the rate seen in studies was “low,” at less than 4 percent.
In the new study, the rate of heart attacks and deaths from cardiovascular incidents did not differ significantly between the dosages, the company said.
The new findings could also raise questions about the risks of Avastin, a cancer drug sold by Genentech. It is being used off-label to treat macular degeneration because it is far cheaper than Lucentis, which costs about $2,000 per monthly injection. Both drugs work by a similar mechanism, although Lucentis was specifically designed to be injected into the eye.
Doctors who use Avastin say they have experienced few if any safety problems. But Avastin has not been tested in extensive controlled clinical trials as Lucentis has.
By ANDREW POLLACK
Published: January 27, 2007
LOS ANGELES, Jan. 26 — Use of Genentech’s new eye drug, Lucentis, might raise the risk of stroke, the company said Friday.
The company posted on its Web site a letter it sent to retina specialists this week. According to the letter, 1.2 percent of patients treated with a high dose of Lucentis in a clinical trial suffered a stroke, compared with only 0.3 percent of those treated with a low dose. The difference was statistically significant.
The new findings could conceivably damp some demand for Lucentis, because the high dose, 0.5 milligrams per injection, is the one that is marketed. Shares of Genentech fell 89 cents, or 1 percent, to $86.57.
Dawn Kalmar, a spokeswoman for Genentech, said Friday that the company wanted to move quickly to communicate the information. But she said the company did not expect the new data to prompt a change in the drug’s label, which already contains a warning about risks of blood-clotting events like strokes.
Lucentis, also called ranibizumab, is approved to treat age-related macular degeneration, the leading cause of blindness in the elderly. It is the first drug shown in clinical trials to improve eyesight for a significant number of patients, as opposed to merely slowing the rate of vision loss.
Approved in June, the drug had sales of $380 million for the rest of 2006, an amount that greatly exceeded some analysts’ expectations. Genentech executives have cautioned, though, that sales growth may moderate as patients on the drug are treated less frequently.
The new data was preliminary, from 2,400 patients who had been followed for an average of 230 days since beginning treatment. The full study will continue and will follow them for a year.
Dr. Philip J. Rosenfeld, a retina specialist, said the preliminary data would not influence him because it could later change.
“Right now, it will have no impact on my use of Lucentis,” Dr. Rosenfeld, who is at the Bascom Palmer Eye Institute of the University of Miami, said in an e-mail message. “When given a choice between a high likelihood of blindness or a 1 percent risk of stroke, I think most patients will choose their vision.”
The label for Lucentis notes a theoretical risk of blood-clotting events like strokes and heart attacks, though it says the rate seen in studies was “low,” at less than 4 percent.
In the new study, the rate of heart attacks and deaths from cardiovascular incidents did not differ significantly between the dosages, the company said.
The new findings could also raise questions about the risks of Avastin, a cancer drug sold by Genentech. It is being used off-label to treat macular degeneration because it is far cheaper than Lucentis, which costs about $2,000 per monthly injection. Both drugs work by a similar mechanism, although Lucentis was specifically designed to be injected into the eye.
Doctors who use Avastin say they have experienced few if any safety problems. But Avastin has not been tested in extensive controlled clinical trials as Lucentis has.
Médicos cubanos na Venezuela: uma relação de trabalho bem conhecida.
O Estado de S. Paulo publica reportagem de Paulo Moreira Leite sobre os médicos cubanos na Venezuela. Vale a pena ler e guardar. Do ponto de vista estritamente técnico, as melhorias apresentadas são questionáveis, mas não tenho o relato completo para avaliar. Do ponto de vista dos direitos humanos, o texto do artigo de que merece muita reflexão é apresentado logo a seguir apresentado em negrito:
Mais de 80% dos custos da Missão Bairro Adentro, como o programa é chamado, são bancados diretamente pela PDVSA, a estatal de petróleo. Os médicos cubanos recebem salários que são uma miséria em Caracas - mas equivalem a rendimentos polpudos em Havana. Um médico cubano embolsa um modestíssimo salário mínimo mensal em Caracas (pouco mais de US$ 250) e uma ajuda anual de US $ 600 para seus familiares, que permanecem em Cuba. (Na ilha, um ótimo salário fica em torno de US$ 50 por mês). Os rendimentos dos cubanos explicam a rotina de sacrifícios a que são submetidos na Venezuela, onde não têm direito a recusar atendimento mesmo quando devem visitar um doente em casa, de madrugada. Muitos residem em casas coletivas onde se divide o trabalho de lavar roupa, cozinhar e varrer o chão. Nos fins de semana, os cubanos comparecem a cursos de atualização. Só passeiam em grupos, num regime de vigilância permanente que sugere o receio de que possam fugir do esquema e pedir asilo, como faziam tantos esportistas nos tempos da Guerra Fria. Aldo Muñoz, o vice-ministro da Saúde de Cuba, mudou-se para Caracas, onde mantém e dirige uma estrutura paralela que controla todos os profissionais estabelecidos no país, define seus passos, autoriza deslocamentos e define atividades nas horas de folga.
Como se define alguém que ganha menos do que o mercado de trabalho porque a família se encontra em outro país sem poder emigrar com regime de trabalho? Onde estão as ONGs e a Organização Internacional do Trabalho? O link do artigo é
http://txt.estado.com.br/editorias/2007/01/27/int-1.93.9.20070127.7.1.xmlOs médicos cubanos já encantaram aqui vários governantes, municipais, estaduais e federais há mais de uma década. Hoje, estão em baixa, tanto pela reação do Conselho Federal de Medicina como pela eficiência baixa desses médicos. A luta contra a efetivação desses profissionais foi chamada de "corporativista" por várias autoridades contrariadas em poder "controlar" os médicos tal como os venezuelanos fazem. Ainda bem que há corporações funcionando no país.
Medicamentos de alto custo: o cinismo a toda prova.
Valor Econômico publicou reportagem de André Vieira em 26/01/07 que pode ser considerada para o prêmio "curto e grosso". Poucas palavras foram reproduzidas de dirigentes da Big Pharma no Brasil, mas o suficiente para mostrar a farra que se vive aqui onde o orçamento de medicamentos de alto custo aumenta em taxa maior (16% entre 2006 e 2007) que os demais itens orçamentários. Um dirigente afirma que distribui gratuitamente o "seu" medicamento por 45 dias e, depois os pacientes conseguem o recebimento por via judicial. Bem, paro por aqui porque dá raiva saber que não recurso para atender a doença que mais mata os brasileiros, mas se paga o tratamento de doenças raras que não são cobertas na maioria dos países ricos. Quem puder leia a reportagem completa sobre esse setor da economia de 1,5 bilhão de reais que envolve médicos, advogados, ONGs e, obviamente os dirigentes da Big Pharma. Quem não tiver acesso, posso enviar uma cópia da reportagem.
Cartel dos Gases Hospitalares: transcrição sem comentários.
Na Folha de S. Paulo, 26 de janeiro de 2007:
A Secretaria de Direito Econômico, órgão do Ministério da Justiça, concluiu que as cinco principais empresas fornecedoras de gases industriais para hospitais públicos e particulares (como oxigênio usado no tratamento de pacientes) formaram um cartel, repartiram o mercado brasileiro entre si e combinaram preços mais altos.São acusadas a White Martins S/A, Aga S/A, a Air Products, a IBG (Indústria Brasileira de Gases) e a Air Liquide.Depois de três anos de investigação, a SDE recomendou ontem a aplicação de uma "multa exemplar" às empresas -o que pode ultrapassar R$ 640 milhões no caso da White Martins, líder do mercado. As 2.700 páginas do processo serão agora analisadas pelo Cade (Conselho Administrativo de Defesa Econômica), que decidirá se e como elas serão punidas.Além das empresas, os executivos podem receber multas. Neste caso, a punição varia de 20% a 50% do valor que a companhia for condenada a pagar."O objetivo era evitar a competição aguerrida entre as empresas. E há indícios da participação direta dos executivos", afirmou a secretária interina Mariana Tavares, ontem.A SDE não calculou o prejuízo causado aos cofres públicos ou a porcentagem de sobrepreço cobrada pelo cartel. Mas a secretária Tavares admitiu que praticamente todas as licitações de Estados e municípios de 2001 a 2003 foram afetadas.O caso foi considerado tão grave e sofisticado pela secretaria que, pela primeira vez, foi recomendada multa exemplar.
sexta-feira, 26 de janeiro de 2007
Derrame, acidente vascular cerebral ou encefálico: não interessa o nome, somente um problema de saúde pública.
Essa semana The Lancet publica uma edição ao acidente vascular cerebral. Brasil é um dos países com as maiores taxas de mortalidade no mundo. Já publiquei editorial afirma que essa, sim é uma doença negligenciada. Nesses artigos há pontos importantes que podem melhorar a qualidade do atendimento, mas com custo aumentado.
(1) diagnóstico: o uso da ressonância nuclear magnética aumenta a capacidade diagnóstica comparada ao da tomografia computadorizada;
(2) tratamento: o uso da alteplase com trombolítico na fase aguda mostrou-se benéfica mesmo em hospitais regionais . A alteplase é mais cara que a estreptoquinase que é utilizada largamente no infarto agudo do miocárdio. A estreptoquinase é contra-indicada na isquemia cerebral;(3) internação: as unidades de avc, chamadas de stroke unit mostram uma relação custo-benefício favorável porque melhoram a qualidade do atendimento da mesma forma como as unidades coronarianas se consagraram há 40 anos como local e forma de atendimento ao infarto do miocárdio.
Outros textos recentes estão mostrando que a relação custo-benefício do tratamento da hipertensão, o principal fator de risco para o acidente vascular cerebral, é melhor do que o tratamento da tuberculose.
Abaixo, trechos do editorial do The Lancet.A favourite interview question put to aspiring doctors applying to be a Lancet editor is: “How many people do you think die each year?” For readers familiar with global burden of disease statistics, this might appear to be an easy question. But for many doctors, who perhaps have not been required to think globally before, it can prove to be a difficult one to answer. Most candidates know that there are around 6·5 billion people living on the planet, so those who guess that around 1% of the population dies each year are not far wrong—around 59 million people will die in 2007. But, perhaps surprisingly, one disease—stroke—will kill 10% of these and leave millions of others disabled.
Unfortunately, despite years of research, alteplase is still the only approved treatment for ischaemic stroke. More than a decade has passed since the publication of the first trial showing that alteplase is efficacious in some patients if given within 3 h of stroke onset. But even now only a tiny proportion of patients who could benefit are given the drug. As the investigators of the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) show in an Article, substantial evidence now exists that intravenous alteplase is safe and effective, even when administered in hospitals with relatively little previous experience of thrombolytic therapy for stroke. However, most stroke researchers would agree that dedicated stroke centres are needed to deliver the best quality of care, a conclusion that is supported by research done by Italian investigators in an Article on page 299. Ideally, a stroke centre will have access to MRI, which, according to Julio Chalela and colleagues, is better than computed tomography for detection of acute ischaemia, and is also able to detect acute and chronic haemorrhage.
Libia: Gaddafi transformou médico e enfermeiras em reféns.
Esse blog talvez seja o único a se preocupar com a situação do médico palestino e das enfermeiras búlgaras presos na Líbia sob a acusação de terem contaminados crianças com o HIV em um hospital. Agora, na reportagem do The Lancet, fica evidente o caráter de retaliação do ditador líbio ao investir contra esses profissionais de saúde.
Libya bargains with lives of imprisoned medics
Katy Duke , The Lancet, 27/01/07
Six medics remain sentenced to death in Libya, accused of deliberately infecting hundreds of children with HIV in a city hospital. Libya's leader, Muammar Gaddafi, is attempting to trade their lives for one of the country's agents, currently serving life in prison in the UK. Katy Duke reports.
Five Bulgarian nurses and a Palestinian doctor were controversially convicted late last year of deliberately infecting hundreds of children with the HIV virus at a Libyan hospital—a ruling that brought condemnation from the international community.
Despite repeated and intense pressure for their release from Bulgarian and other officials, who claim the six are innocent, the Libyan leader, Muammar Gaddafi, appears to be now using them as pawns in a political game.
During a speech to officials in Tripoli at the end of last year Gaddafi said that he would only consider repealing a death sentence on the six medical staff if the UK agreed to release the Libyan agent sentenced to life in prison for putting a bomb on the Pan Am flight that exploded over the Scottish village of Lockerbie in 1988.
Katy Duke , The Lancet, 27/01/07
Six medics remain sentenced to death in Libya, accused of deliberately infecting hundreds of children with HIV in a city hospital. Libya's leader, Muammar Gaddafi, is attempting to trade their lives for one of the country's agents, currently serving life in prison in the UK. Katy Duke reports.
Five Bulgarian nurses and a Palestinian doctor were controversially convicted late last year of deliberately infecting hundreds of children with the HIV virus at a Libyan hospital—a ruling that brought condemnation from the international community.
Despite repeated and intense pressure for their release from Bulgarian and other officials, who claim the six are innocent, the Libyan leader, Muammar Gaddafi, appears to be now using them as pawns in a political game.
During a speech to officials in Tripoli at the end of last year Gaddafi said that he would only consider repealing a death sentence on the six medical staff if the UK agreed to release the Libyan agent sentenced to life in prison for putting a bomb on the Pan Am flight that exploded over the Scottish village of Lockerbie in 1988.
quinta-feira, 25 de janeiro de 2007
Plano Bush na saúde: mais atrapalha do que ajuda.
Analisei um pouco mais a proposta Bush para a atenção médica. Incrível, mas o "país com o melhor sistema de saúde e atenção médica do mundo" (Bush) está estimulando os planos individuais. Aqui, no Brasil, onde esquerdistas (versão sindical) e direitistas (versão corporativa) adoram falar na "vala comum do SUS" comprova-se dia a dia que planos individuais estão fadados ao fracasso, a não ser quando os valores dos prêmios são elevados, fora da possibilidade dos mortais. A solução são planos nacionais bem orçamentados com administração descentralizada, como cada vez mais se comprova desde a implantação do SUS, tanto pelos acertos, como pelos erros. Voltando aos EUA, O plano Bush irá atrapalhar as propostas de estados como a California que estão estimulando os empregadores por meio de subsídios. Lembro que Mitt Rommey, ex-governador de Masschusetts é candidato à Presidência pelo Partido Republicano e, criticou também a proposta. Se a questão Iraque estiver "resolvida" até 2008, Rommey será páreo duro aos democratas com a sua proposta na área da saúde.
Maiores detalhes na reportagem de hoje do The Wall Street Journal.
Bush Health Plan Shifts Onus to the Consumer
Proposal Contrasts EffortsBy States, Democrats to Push Employers to Secure Coverage
By JANE ZHANG and SARAH LUECKJanuary 25, 2007; Page A6
President Bush's proposal for easing the nation's health-care woes -- encouraging families to buy insurance on their own instead of relying on employer-provided insurance -- differs from approaches favored by Massachusetts and California as well as many Democrats in Congress.
In their bids to extend health-care coverage to millions of uninsured, former Massachusetts Gov. Mitt Romney and California Gov. Arnold Schwarzenegger, both Republicans, aim to shore up existing coverage, which has been offered by fewer employers in recent years. The Massachusetts plan, which was supported by Democratic Sen. Edward Kennedy, requires all but the smallest employers to offer health insurance, or else pay an assessment to the state. In California, a similar requirement would compel employers that don't offer insurance to pay a 4% payroll tax.
WALL STREET JOURNAL VIDEO
President Bush outlines a new tax deduction for families and individuals with private health insurance and other health initiatives. Plus, Health-care policy expert Dr. Paul Ginsburg speaks with the Journal's Matt Murray about how Bush's health-care reform would be funded.
Mr. Bush, in contrast, avoids mandates and instead would use the tax code to make individually purchased health insurance more appealing and affordable. In a break with the past, he would make employer-provided health insurance taxable, but create a tax deduction of $15,000 for families and $7,500 for singles, for everyone who gets insurance, through work or on the open market. That is designed, the White House says, to be fair -- by treating individuals who buy insurance and now receive no tax break, the same as employees who are covered through their work and can exclude the value of the insurance from taxable income. Another part of the president's plan would help states extend coverage to the uninsured by diverting money from hospitals that care for the poor into state insurance pools.
"What you are seeing is indicative of sharp philosophical differences," said Robert Laszewski, a health-policy analyst. "One is to build on the employer-based system that most Democrats embrace and the other is to develop an entirely new system controlled by the consumer, not the employer," which many -- though far from all -- Republicans and the president endorse.
Both states would require all residents to have health insurance, offering government coverage or subsidies to those who are without coverage through an employer.
Whether Mr. Bush's plan will contribute to a further erosion of employer-sponsored health coverage was debated yesterday. Health and Human Services Secretary Michael Leavitt told reporters that the president's plan will "absolutely not" have a negative effect on the employer-based system. Rather, he said, it does something that states can't: changes the federal tax code to help individuals buy health insurance. "I do not see employers leaving the employment-based system," he said.
Not everyone in the administration agreed. At a White House briefing on Tuesday, Joel Kaplan, deputy chief of staff for policy, acknowledged that the proposal could accelerate the trend of employers dropping insurance, but emphasized that workers left without coverage would, thanks to the new tax deduction, have the means to "buy insurance in the individual market in a way that they can't now."
Some employer and industry groups fretted about the proposal. Neil Trautwein, vice president of the National Retail Federation, said, "The fear is that if it becomes more attractive for people to decline employer coverage and buy on the open market, then you could get great spirals in the employer plans. I'm not convinced we should take away one of the pillars that has been supporting the health-care system."
Democratic lawmakers said that expanding government programs, not changing the tax code, is a cheaper, simpler way to lower the ranks of uninsured than relying on the individual market, where policies often are expensive and people with health problems have trouble buying coverage at any price. "With the individual market, people pay more for [policies]; they also pay for insurance company overhead. And [private insurers] traditionally discriminate against people with prior medical problems," said Rep. Henry Waxman, a California Democrat and a senior member of a House committee that oversees health-care issues.
Nina Owcharenko, a senior policy analyst for health care at the Heritage Foundation, said the Bush plan would complement states' effort to expand coverage: "The states control the regulatory structure of the individual market," especially for small businesses and individuals, and "this proposal is saying, 'How do we work together?' "
Ms. Owcharenko added: "This is trying to find a way to make sure there's a market for an individual so they can buy health insurance. Both can co-exist.
"It's not removing the employer system. It's not keeping the individual market as it is," she said. "The overall impact is combining the tax code with state innovations. The end goal will be to increase the number of people who have private insurance."
Proposal Contrasts EffortsBy States, Democrats to Push Employers to Secure Coverage
By JANE ZHANG and SARAH LUECKJanuary 25, 2007; Page A6
President Bush's proposal for easing the nation's health-care woes -- encouraging families to buy insurance on their own instead of relying on employer-provided insurance -- differs from approaches favored by Massachusetts and California as well as many Democrats in Congress.
In their bids to extend health-care coverage to millions of uninsured, former Massachusetts Gov. Mitt Romney and California Gov. Arnold Schwarzenegger, both Republicans, aim to shore up existing coverage, which has been offered by fewer employers in recent years. The Massachusetts plan, which was supported by Democratic Sen. Edward Kennedy, requires all but the smallest employers to offer health insurance, or else pay an assessment to the state. In California, a similar requirement would compel employers that don't offer insurance to pay a 4% payroll tax.
WALL STREET JOURNAL VIDEO
President Bush outlines a new tax deduction for families and individuals with private health insurance and other health initiatives. Plus, Health-care policy expert Dr. Paul Ginsburg speaks with the Journal's Matt Murray about how Bush's health-care reform would be funded.
Mr. Bush, in contrast, avoids mandates and instead would use the tax code to make individually purchased health insurance more appealing and affordable. In a break with the past, he would make employer-provided health insurance taxable, but create a tax deduction of $15,000 for families and $7,500 for singles, for everyone who gets insurance, through work or on the open market. That is designed, the White House says, to be fair -- by treating individuals who buy insurance and now receive no tax break, the same as employees who are covered through their work and can exclude the value of the insurance from taxable income. Another part of the president's plan would help states extend coverage to the uninsured by diverting money from hospitals that care for the poor into state insurance pools.
"What you are seeing is indicative of sharp philosophical differences," said Robert Laszewski, a health-policy analyst. "One is to build on the employer-based system that most Democrats embrace and the other is to develop an entirely new system controlled by the consumer, not the employer," which many -- though far from all -- Republicans and the president endorse.
Both states would require all residents to have health insurance, offering government coverage or subsidies to those who are without coverage through an employer.
Whether Mr. Bush's plan will contribute to a further erosion of employer-sponsored health coverage was debated yesterday. Health and Human Services Secretary Michael Leavitt told reporters that the president's plan will "absolutely not" have a negative effect on the employer-based system. Rather, he said, it does something that states can't: changes the federal tax code to help individuals buy health insurance. "I do not see employers leaving the employment-based system," he said.
Not everyone in the administration agreed. At a White House briefing on Tuesday, Joel Kaplan, deputy chief of staff for policy, acknowledged that the proposal could accelerate the trend of employers dropping insurance, but emphasized that workers left without coverage would, thanks to the new tax deduction, have the means to "buy insurance in the individual market in a way that they can't now."
Some employer and industry groups fretted about the proposal. Neil Trautwein, vice president of the National Retail Federation, said, "The fear is that if it becomes more attractive for people to decline employer coverage and buy on the open market, then you could get great spirals in the employer plans. I'm not convinced we should take away one of the pillars that has been supporting the health-care system."
Democratic lawmakers said that expanding government programs, not changing the tax code, is a cheaper, simpler way to lower the ranks of uninsured than relying on the individual market, where policies often are expensive and people with health problems have trouble buying coverage at any price. "With the individual market, people pay more for [policies]; they also pay for insurance company overhead. And [private insurers] traditionally discriminate against people with prior medical problems," said Rep. Henry Waxman, a California Democrat and a senior member of a House committee that oversees health-care issues.
Nina Owcharenko, a senior policy analyst for health care at the Heritage Foundation, said the Bush plan would complement states' effort to expand coverage: "The states control the regulatory structure of the individual market," especially for small businesses and individuals, and "this proposal is saying, 'How do we work together?' "
Ms. Owcharenko added: "This is trying to find a way to make sure there's a market for an individual so they can buy health insurance. Both can co-exist.
"It's not removing the employer system. It's not keeping the individual market as it is," she said. "The overall impact is combining the tax code with state innovations. The end goal will be to increase the number of people who have private insurance."
Dirigindo as Diretrizes
O The New England Journal of Medicine publica o artigo Guidance for Guidelines de Robert Steinbrook, questionando os famosos "guidelines" ou diretrizes. Obviamente, o problema maior é a ligação estreita dos autores de diretrizes com a indústria farmacêutica e de equipamentos. Eu não gosto das diretrizes, por esse aspecto e, também por transformar a medicina em prática chata e burocrática. Posso parecer contraditório, mas as diretrizes são muito mais apropriadas às práticas de saúde pública como vacinação, check-up, atendimento de emergência do que à clínica do dia a dia. Abaixo, trecho do texto de Steinbrook.
Guidelines have also been questioned when pharmaceutical and medical-device companies with a financial stake in the outcome provide substantial funding for their development and implementation. When members of guideline committees also have substantial financial associations with industry, further questions inevitably arise. Some argue that public disclosure of sponsorship and of the financial associations of committee members, along with rules to prevent sponsors from influencing the selection of panel members and the content of guidelines, are adequate safeguards. Others maintain that practice recommendations will invariably be viewed with skepticism unless corporate sponsorship and experts with financial ties are completely avoided.
At present, the financial ties between guidelines panels and industry are extensive. A survey of 685 disclosure statements by authors of guidelines concerning medications found that 35% declared a potential financial conflict of interest. In 2006, Eli Lilly was criticized for providing the impetus for the development of practice guidelines for sepsis treatment and coordinating the process with a marketing campaign for Xigris (recombinant human activated protein C). And Amgen and other companies that manufacture or market recombinant erythropoietin, as well as DaVita, a large company that provides dialysis services, have been criticized for their close relations to the development of the National Kidney Foundation's guidelines for managing anemia in chronic kidney disease. An alternative approach is government sponsorship, although it does not ensure that committee members are independent of commercial interests. In 2004, the National Cholesterol Education Program updated its guidelines for the detection, evaluation, and treatment of high blood cholesterol in adults. It was subsequently disclosed that most of the committee members had extensive financial connections to the manufacturers of statins, which stood to gain from increased use of these drugs.
At present, the financial ties between guidelines panels and industry are extensive. A survey of 685 disclosure statements by authors of guidelines concerning medications found that 35% declared a potential financial conflict of interest. In 2006, Eli Lilly was criticized for providing the impetus for the development of practice guidelines for sepsis treatment and coordinating the process with a marketing campaign for Xigris (recombinant human activated protein C). And Amgen and other companies that manufacture or market recombinant erythropoietin, as well as DaVita, a large company that provides dialysis services, have been criticized for their close relations to the development of the National Kidney Foundation's guidelines for managing anemia in chronic kidney disease. An alternative approach is government sponsorship, although it does not ensure that committee members are independent of commercial interests. In 2004, the National Cholesterol Education Program updated its guidelines for the detection, evaluation, and treatment of high blood cholesterol in adults. It was subsequently disclosed that most of the committee members had extensive financial connections to the manufacturers of statins, which stood to gain from increased use of these drugs.
Open Letter to Academic Medical Leaders
Lançado hoje no British Medical Journal, a carta aberta às lideranças médicas no mundo todo. O acesso é livre no link abaixo.
Apresentarei algumas partes com comentários nos próximos dias.
quarta-feira, 24 de janeiro de 2007
A nova proposta de Bush na área da saúde e de ambiente
Abaixo a nova proposta da administração Bush apresentada ontem, dia 23/01/07 no Congresso sobre saúde. A frase provoca riso: os americanos têm a sorte de ter o mais avançado e inovador sistema de saúde do mundo. Mas, no restante representa um recuo das posições anteriores do Partido Republicano nessa área, bem como na questão ambiental. A taxa de aprovação de 21%, como divulgado antes da fala presidencial, induziu a mudança de pontos programáticos.
A proposta de redução da dependência da gasolina e diesel e, substituição por fontes alternativas terá impacto (positivo?) na economia brasileira e (negativo?) no ambiente e, merece análise séria por especialistas em economia e ambiente.
Health Care
Americans are fortunate to have the most advanced and innovative health care system in the world.
Americans are fortunate to have the most advanced and innovative health care system in the world.
The President's Plan Includes Two Parts: Reforming The Tax Code With A Standard Deduction For Health Insurance So All Americans Get The Same Tax Breaks For Health Insurance And Helping States Make Affordable Private Health Insurance Available To Their Citizens.
1. The President's Plan Will Help More Americans Afford Health Insurance By Reforming The Tax Code With A Standard Deduction For Health Insurance – Like The Standard Deduction For Dependents. The President's primary goal is to make health insurance more affordable, allowing more Americans to purchase coverage. The 1/23/07 White House Office Of Communications President's proposal levels the playing field for Americans who purchase health insurance on their own rather than
through their employers, providing a substantial tax benefit for all those who now have health insurance purchased on the individual market. It also lowers taxes for all currently uninsured Americans who decide to purchase health insurance – making insurance more affordable and providing a significant incentive to all working Americans to purchase coverage, thereby reducing the number of uninsured Americans.
! Under The President's Proposal, Families With Health Insurance Will Not Pay Income Or Payroll Taxes On The First $15,000 In Compensation And Singles Will Not Pay Income Or Payroll Taxes On The First $7,500.
o At the same time, health insurance would be considered taxable income. This is a change for those who now have health insurance through their jobs. o The President's proposal will result in lower taxes for about 80 percent of employer-provided policies. Those with more generous policies (20 percent) will have the option to adjust their compensation to have lower premiums and higher wages to offset the tax change.
2. The President's Affordable Choices Initiative Will Help States Make Basic Private Health Insurance Available And Will Provide Additional Help To Americans Who Cannot Afford Insurance Or Who Have Persistently High Medical Expenses. For States that provide their citizens with access to basic, affordable private health insurance, the President's Affordable Choices Initiative will direct Federal funding to assist States in helping their poor and hard-to-insure citizens afford private insurance. By allocating current Federal health care funding more effectively, the President's plan accomplishes this goal without creating a new Federal entitlement or new Federal spending. These Two Policies Will Work Together To Help More Americans Afford Basic Private Coverage. The President's proposed standard deduction for health insurance will help make basic private health insurance more affordable for families and individuals – whether they have insurance through their jobs or purchase insurance on their own. For those who remain unable to afford coverage, the President's Affordable Choices Initiative will help eligible States assist their poor and hard-toinsure citizens in purchasing private health insurance. There Are Many Other Ways That Congress Can Help. We need to expand Health Savings Accounts, help small businesses through Association Health Plans, reduce costs and medical errors with better information technology, encourage price transparency, and protect good doctors from predatory lawsuits by passing medical liability reform.
1. The President's Plan Will Help More Americans Afford Health Insurance By Reforming The Tax Code With A Standard Deduction For Health Insurance – Like The Standard Deduction For Dependents. The President's primary goal is to make health insurance more affordable, allowing more Americans to purchase coverage. The 1/23/07 White House Office Of Communications President's proposal levels the playing field for Americans who purchase health insurance on their own rather than
through their employers, providing a substantial tax benefit for all those who now have health insurance purchased on the individual market. It also lowers taxes for all currently uninsured Americans who decide to purchase health insurance – making insurance more affordable and providing a significant incentive to all working Americans to purchase coverage, thereby reducing the number of uninsured Americans.
! Under The President's Proposal, Families With Health Insurance Will Not Pay Income Or Payroll Taxes On The First $15,000 In Compensation And Singles Will Not Pay Income Or Payroll Taxes On The First $7,500.
o At the same time, health insurance would be considered taxable income. This is a change for those who now have health insurance through their jobs. o The President's proposal will result in lower taxes for about 80 percent of employer-provided policies. Those with more generous policies (20 percent) will have the option to adjust their compensation to have lower premiums and higher wages to offset the tax change.
2. The President's Affordable Choices Initiative Will Help States Make Basic Private Health Insurance Available And Will Provide Additional Help To Americans Who Cannot Afford Insurance Or Who Have Persistently High Medical Expenses. For States that provide their citizens with access to basic, affordable private health insurance, the President's Affordable Choices Initiative will direct Federal funding to assist States in helping their poor and hard-to-insure citizens afford private insurance. By allocating current Federal health care funding more effectively, the President's plan accomplishes this goal without creating a new Federal entitlement or new Federal spending. These Two Policies Will Work Together To Help More Americans Afford Basic Private Coverage. The President's proposed standard deduction for health insurance will help make basic private health insurance more affordable for families and individuals – whether they have insurance through their jobs or purchase insurance on their own. For those who remain unable to afford coverage, the President's Affordable Choices Initiative will help eligible States assist their poor and hard-toinsure citizens in purchasing private health insurance. There Are Many Other Ways That Congress Can Help. We need to expand Health Savings Accounts, help small businesses through Association Health Plans, reduce costs and medical errors with better information technology, encourage price transparency, and protect good doctors from predatory lawsuits by passing medical liability reform.
terça-feira, 23 de janeiro de 2007
Uma ótima proposta: o propagandista acadêmico de medicamentos.
Abaixo, o texto de Jerry Avorn, professor titular de atenção primária da Harvard propondo que governos montem esquemas de propaganda da boa prática médica tal qual os laboratórios fazem há décadas.
When I was practicing primary care, each year it got tougher to stay on top of the medical literature. But I had unlimited access to dozens of attractive, articulate people who came right to my office, at my convenience, to "update" me on topics like "modern concepts in hypertension" or "how to lower your patients' cholesterol." Some brought me lunch while they taught me; others offered to provide the education at a Red Sox game. The material they provided was user-friendly, accessible, engaging, and clinically relevant. But the only reason these "detail men or detail women" did this was to increase the sales of products of the drug companies each of them worked for, and the data they presented were carefully selected to accomplish just that goal. I wished that someone would come to my office and give me user-friendly drug information that was more neutral, not designed primarily to push products.
To address that unmet need, my colleagues and I created the concept of "academic detailing." We trained pharmacists to visit doctors in their offices to offer concise, clinically relevant overviews of various therapeutic categories. The only goal was to provide unbiased, commercial-free data about optimal care.[1] Sometimes they even brought lunch.
The original program was funded by a federal grant, but today similar programs are in operation in several countries.[2] In Australia, the government pays for a nationwide program that covers 60% of the country's primary care doctors. We've recently begun a program in Pennsylvania funded by that state; our reviews and teaching materials are freely available to all at www.RxFacts.org.[3] We all benefit if doctors have access to even-handed, non-product-driven drug information. Such programs can improve appropriate prescribing, and over time should even be able to save more than they cost, while helping us make smarter prescribing choices for our patients.
That's my opinion. I'm Dr. Jerry Avorn, Professor of Medicine at Harvard Medical School.
Sign Up now for a free monthly email that brings you the top features from MedGenMed.Readers are encouraged to respond to the author at javorn@partners.org or to George Lundberg, MD, Editor of MedGenMed, for the editor's eyes only or for possible publication via email: glundberg@medscape.net
To address that unmet need, my colleagues and I created the concept of "academic detailing." We trained pharmacists to visit doctors in their offices to offer concise, clinically relevant overviews of various therapeutic categories. The only goal was to provide unbiased, commercial-free data about optimal care.[1] Sometimes they even brought lunch.
The original program was funded by a federal grant, but today similar programs are in operation in several countries.[2] In Australia, the government pays for a nationwide program that covers 60% of the country's primary care doctors. We've recently begun a program in Pennsylvania funded by that state; our reviews and teaching materials are freely available to all at www.RxFacts.org.[3] We all benefit if doctors have access to even-handed, non-product-driven drug information. Such programs can improve appropriate prescribing, and over time should even be able to save more than they cost, while helping us make smarter prescribing choices for our patients.
That's my opinion. I'm Dr. Jerry Avorn, Professor of Medicine at Harvard Medical School.
Sign Up now for a free monthly email that brings you the top features from MedGenMed.Readers are encouraged to respond to the author at javorn@partners.org or to George Lundberg, MD, Editor of MedGenMed, for the editor's eyes only or for possible publication via email: glundberg@medscape.net
Carta ao secretário de saúde do Rio de Janeiro
Prezado Sérgio Côrtes, admirei seu trabalho no Into. Poucos dirigentes hospitalares tiveram coragem de enfrentar o esquema das "próteses-órteses". Dos que "peitaram" esse esquema, a maioria o fez em silêncio, mas você foi obrigado a se manifestar em auto-defesa pessoal e familiar. Entendo sua atitude. Desejo boa sorte na Secretaria de Estado, porque competência não lhe falta. Acuse à vontade, o governador e secretário anteriores, porque eles sabem se defender e, com certeza revidarão. Mas, por favor, não desqualique médicos e enfermeiros como na entrevista que li no Estadão de hoje. Se você quiser ter uma boa gestão, somente a fará com profissionais motivados e respeitados. O seu chefe, o Governador Sérgio Cabral, já deu um mal exemplo em falar coisas como "genocídio" e, acusar injustamente um hospital pela morte da empregada doméstica. Por fim, você caiu um pouco no conto do "8 ou 800". Não sei quanto um hospital precisa de despesas extraordinárias por mês, afinal depende do porte e complexidade, mas valorizar os 8 mil reais dos hospitais federais para uso contigencial, ofende a inteligência dos demais administradores hospitalares do país.
Lembre que sou de São Paulo, não voto no Rio e, não tenho nenhuma simpatia pelos governos que o antecederam.
Atenciosamente
Paulo Lotufo
Pfizer corta 10 mil funcionários no mundo
A maior da Big Pharma foi atingidia pela queda de patentes, redução da venda do Viagra e, por muito mais coisa que não divulgam. Apesar das críticas constantes à Big Pharma, o fato da maior empresa estar reduzindo gastos e cortando pessoal é muito ruim. Espero, sinceramente que os gastos sejam na parte pior dessas empresas:o marketing e os sales rep. E, em oposição, o setor de desenvolvimento seja mantido. Há muito tempo defendo que o marketing gasta mais do de arrecada de fato. O problema é que os marqueteiros são bons, principalmente, em fazer a propaganda deles próprios. Se as farmacêuticas tivessem mais ouvidos e olhos epidemiológicos do que a boca marqueteira, talvez estivessem em situação mais estável. Vide o exemplo anterior do lupus eritematoso que foi desconsiderado pela indústria por décadas.
Lupus: a Big Pharma acordou
Essa notícia relaciona-se a uma conversa com uma ex-residente que deve estar no momento acertando sua vida em uma nova casa no exterior onde estagiará em serviço especializado em lupus eritematoso. Concluímos que as pesquisas com lúpus não avançaram porque a indústria farmacêutica não tinha opção alguma ao conhecido esquema corticóide e imunossupressor.
O lupus é uma doença muito comum entre as brasileiras, acredito que com prevalência superior à da população americana. Agora, a Big Pharma acordou do erro em não dedicar pesquisa específica ao lupus eritematoso. Além do erro da taxa de prevalência que o artigo abaixo alega, também há uma hipótese sobre a distribuição social da doença lúpica, que seria muito mais frequente em mulheres pobres.
Abaixo, o parte de artigo do The Wall Street Journal de hoje;
Drugs in Testing Show Promise for Lupus
New Treatments TargetDisease, Not Just Symptoms;First Big Advances in 50 Years
By HEATHER WON TESORIEROJanuary 23, 2007; Page D1
Several drug makers are in advanced-stage trials for lupus drugs. Human Genome Sciences Inc. will begin enrolling patients in the next two weeks in the largest ever late-stage lupus trial, following positive results in earlier testing; Bristol-Myers Squibb Co. is conducting lupus trials on Orencia, its rheumatoid arthritis drug; and Genentech Inc. and Biogen Idec Inc. are conducting late-stage trials on Rituxan, a cancer drug that has been used off-label for lupus. See what lupus treatments are available and what's in the pipeline.
For many years, lupus wasn't well understood by doctors, and can still be difficult to diagnose. There has been no drug that targets the disease itself, and doctors have been able to only treat complications.
Growing Market
With the lack of remedies for the disorder, the government began investing more heavily in lupus research and now spends roughly $89 million a year on the disease. As results emerged, doctors and researchers saw clues into how lupus works in the body's cells. And, while no reliable numbers on growth exist -- a deficit Dr. Lim's patient registry aims to fill -- experts agree the lupus population has expanded along with better diagnoses. In turn, some drug companies began to pay more attention to the disorder.
"Drug companies weren't interested in it because they thought it was a small market," Gary Gilkeson, head of the medical and scientific board of the Lupus Foundation of America, says. "They realized that was wrong." By one estimate, the lupus drug market, which was $300 million in 2005, could reach $1.3 billion in 2015 if the potential treatments prove to be efficacious.
There's now a horserace among a few drug companies to be the first one in 50 years to gain approval to make and market a lupus drug. There are currently four drugs in late-stage lupus trials, as well as two being tested for lupus nephritis. Most of these drugs are monoclonal antibodies, which attempt to target the cells that contribute to the damaging antibodies. Early results for a trial for Lymphostat-B, a collaboration between Human Genome Sciences and GlaxoSmithKline PLC, showed to reduce lupus disease activity. Now, the new international trial will enroll more than 1,600 patients to try and confirm the findings.
Bristol-Myers is conducting late-stage trials with Orencia, its rheumatoid arthritis drug that came out in the U.S. last year, to see if it helps lupus patients. The international trial will enroll 180 to 190 patients. Orencia, which is administered about every 28 days via a 30-minute intravenous infusion, targets T-cells, which are believed to have a major hand in inflammation.
And Genentech in collaboration with Biogen Idec is going ahead with plans to test Rituxan, a blockbuster cancer and rheumatoid arthritis drug, in lupus patients, both with and without lupus nephritis. The lupus trial will enroll 250 patients and the nephritis trial will enroll 140. Last month, the FDA issued a warning following two deaths from a viral infection of lupus patients using the drug off-label. Doctors and analysts took heed, but say that they're still hopeful that the drug will be approved, noting that because lupus patients have compromised immune systems, it's impossible to determine whether Rituxan was responsible.
Aspreva Pharmaceuticals Corp. is testing anti-organ-rejection treatment CellCept for lupus nephritis. CellCept, a drug for organ transplant recipients, is often prescribed off-label to lupus patients. Results from the first phase of its late-stage trials are expected this year
New Treatments TargetDisease, Not Just Symptoms;First Big Advances in 50 Years
By HEATHER WON TESORIEROJanuary 23, 2007; Page D1
Several drug makers are in advanced-stage trials for lupus drugs. Human Genome Sciences Inc. will begin enrolling patients in the next two weeks in the largest ever late-stage lupus trial, following positive results in earlier testing; Bristol-Myers Squibb Co. is conducting lupus trials on Orencia, its rheumatoid arthritis drug; and Genentech Inc. and Biogen Idec Inc. are conducting late-stage trials on Rituxan, a cancer drug that has been used off-label for lupus. See what lupus treatments are available and what's in the pipeline.
For many years, lupus wasn't well understood by doctors, and can still be difficult to diagnose. There has been no drug that targets the disease itself, and doctors have been able to only treat complications.
Growing Market
With the lack of remedies for the disorder, the government began investing more heavily in lupus research and now spends roughly $89 million a year on the disease. As results emerged, doctors and researchers saw clues into how lupus works in the body's cells. And, while no reliable numbers on growth exist -- a deficit Dr. Lim's patient registry aims to fill -- experts agree the lupus population has expanded along with better diagnoses. In turn, some drug companies began to pay more attention to the disorder.
"Drug companies weren't interested in it because they thought it was a small market," Gary Gilkeson, head of the medical and scientific board of the Lupus Foundation of America, says. "They realized that was wrong." By one estimate, the lupus drug market, which was $300 million in 2005, could reach $1.3 billion in 2015 if the potential treatments prove to be efficacious.
There's now a horserace among a few drug companies to be the first one in 50 years to gain approval to make and market a lupus drug. There are currently four drugs in late-stage lupus trials, as well as two being tested for lupus nephritis. Most of these drugs are monoclonal antibodies, which attempt to target the cells that contribute to the damaging antibodies. Early results for a trial for Lymphostat-B, a collaboration between Human Genome Sciences and GlaxoSmithKline PLC, showed to reduce lupus disease activity. Now, the new international trial will enroll more than 1,600 patients to try and confirm the findings.
Bristol-Myers is conducting late-stage trials with Orencia, its rheumatoid arthritis drug that came out in the U.S. last year, to see if it helps lupus patients. The international trial will enroll 180 to 190 patients. Orencia, which is administered about every 28 days via a 30-minute intravenous infusion, targets T-cells, which are believed to have a major hand in inflammation.
And Genentech in collaboration with Biogen Idec is going ahead with plans to test Rituxan, a blockbuster cancer and rheumatoid arthritis drug, in lupus patients, both with and without lupus nephritis. The lupus trial will enroll 250 patients and the nephritis trial will enroll 140. Last month, the FDA issued a warning following two deaths from a viral infection of lupus patients using the drug off-label. Doctors and analysts took heed, but say that they're still hopeful that the drug will be approved, noting that because lupus patients have compromised immune systems, it's impossible to determine whether Rituxan was responsible.
Aspreva Pharmaceuticals Corp. is testing anti-organ-rejection treatment CellCept for lupus nephritis. CellCept, a drug for organ transplant recipients, is often prescribed off-label to lupus patients. Results from the first phase of its late-stage trials are expected this year
segunda-feira, 22 de janeiro de 2007
Um basta ao sensacionalismo, verifiquem a EC 29
Há um ano, a maioria absoluta dos jornalistas, cronistas e blogueiros se questionados pelos termos abaixo responderiam que
(1) Marcola é a nova revelação do Santos F.C. e, cotado para jogar na Ucrânia:
(2) transponder era o instrumento utilizado pelo Dr McCoy em Jornadas nas Estrelas;
(3) grua é uma ave comum no Pantanal;
Em questões de minutos, todos eles passaram a falar e comentar sobre o indivíduo e os produtos acima como se fossem algo familiar como Ronaldinho Gaúcho, ventilador de teto e pardal.
Como a crise aérea afetou muito São Paulo, mas não foi aqui originada, os ataques figadais à cidade não ocorreram. Mas, agora na tragédia do metrô em Pinheiros, a quantidade de asneiras e ataques à cidade passaram do razoável. Não vou citar o nome dos "respeitáveis jornalistas" que assim agiram, somente vou recomendar o ótimo artigo de Ricardo Kotscho criticando a mania de odiar São Paulo. (http://www.nominimo.com)
Desconfio que os adeptos do governo federal, devido ao fato de São Paulo, Estado e cidade serem governados pela oposição exageram e recalcam o seu ódio à cidade.
A eles e demais, um desafio: descubram, avaliem e verifiquem o que se passa com a EC 29. O que será EC 29?
sábado, 20 de janeiro de 2007
Homícidios em São Paulo: o episódio PCC
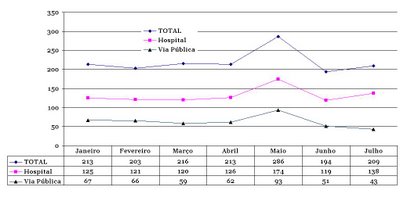 No gráfico ao lado mostro o total de homicídios por mês na cidade de São Paulo no primeiro semestre de 2006, primeiro total, ocorridos em hospital e, depois em via pública. O número de mortes teve um pico no mês de maio, onde houve ataque do PCC a alvos civis e militares na cidade. Nos meses de junho e julho houve queda comparado a janeiro, março e abril. Essa foi a realidade: um episódio triste que afetou inúmeras famílias, a reputação da polícia e permitiu que bandidos de todos os tipos se sentissem fortalecidos. Mas, a tendência de redução do número de homicídios continuou a ocorrer. Em termos restritos, pode-se dizer que o episódio PCC foi realmente "episódico" na tendência histórica.
No gráfico ao lado mostro o total de homicídios por mês na cidade de São Paulo no primeiro semestre de 2006, primeiro total, ocorridos em hospital e, depois em via pública. O número de mortes teve um pico no mês de maio, onde houve ataque do PCC a alvos civis e militares na cidade. Nos meses de junho e julho houve queda comparado a janeiro, março e abril. Essa foi a realidade: um episódio triste que afetou inúmeras famílias, a reputação da polícia e permitiu que bandidos de todos os tipos se sentissem fortalecidos. Mas, a tendência de redução do número de homicídios continuou a ocorrer. Em termos restritos, pode-se dizer que o episódio PCC foi realmente "episódico" na tendência histórica.
Marcadores:
drogas,
homicídios,
mortalidade,
violência
sexta-feira, 19 de janeiro de 2007
Ministério controla o preço da vacina para HPV.
Já mencionei aqui a questão da vacina para o HPV. Com certeza, quem precisará da vacina, não terá acesso e, fará uso quem tem risco mínimo de câncer de colo uterino. É o fenômeno descrito na Inglaterra por Julian Tudor- Hart chamado "inverse care law". O Ministério da Saúde precisa ser rigoroso e, impedir a liberalização total de venda da vacina? Sim, porque (1) somente o Ministério poderá comprar a vacina e, quem tomar em clínica particulares irá deduzir o valor pago no imposto de renda. Ou seja, por compra direta ou por renúncia fiscal, a conta será do erário; (2) existirá em breve, alternativa da Glaxo, que poderá ser mais barata e efetiva. Por isso, se o Ministério facilitar a venda do produto da Merck estará "matando" o concorrente que poderá ser ou não a melhor opção (não há dados confiáveis) entre as duas estratégias de prevenção.
No entanto, a prioridade de aplicação em termos de faixa etária, região e categoria social deveria ser do Ministério. Porque, se com certeza, a moça de 15 anos moradora em Brasília Teimosa em Recife se beneficiará mais da vacina, do que a senhora de 35 anos moradora no plano piloto de Brasília, não preciso ser advinho para saber quem terá e, quem não terá acesso à vacina, casos as "leis de mercado" prevaleçam. Abaixo, um texto elucidativo de Adriana Lopes sobre as vacinas para o HPV, em O Estado de S.Paulo (mas<>
Depois de três meses em estudo, o preço da vacina para o HPV (vírus responsável por 70% dos casos de câncer de colo do útero) acaba de ser definido pela Câmara de Regulação do Mercado de Medicamentos (CMED), órgão do governo federal que regula os preços dos remédios no País: R$ 364,16, a dose. A Merck, laboratório fabricante, entrou com recurso contra o valor.Segundo a Agência Nacional de Vigilância Sanitária (Anvisa), a Merck poderá, mesmo com o recurso, ainda optar por vender ou não o produto, chamado Gardasil, por esse preço.O laboratório irá se manifestar só nos próximos dias. O valor determinado pelo CMED é cerca de metade do que havia pedido o laboratório - entre R$ 500 e R$ 700 a dose.Enquanto isso, a vacina da Merck está prestes a ganhar um concorrente mundial. A Cervarix, da GlaxoSmithKline, deverá ser aprovada pela Anvisa em maio - a previsão é do próprio laboratório, que entrou com pedido de autorização na Anvisa em maio de 2006. A agência sanitária européia (Emea) deve aprovar a vacina para o mercado europeu em março. O preço não foi definido.TRATAMENTOS EXCLUDENTESAs duas vacinas são excludentes, ou seja, a paciente terá de optar por uma delas, e têm diferenças significativas. A principal delas é em relação à faixa etária indicada. Por enquanto, estudos clínicos feitos com a Gardasil mostraram que ela imuniza mulheres de 9 a 26 anos - a eficácia em pacientes mais velhas está em fase de pesquisa.Já a Cervarix será lançada para mulheres de 10 a 55 anos.Há mais de 200 tipos de HPV. A da Merck protege contra quatro - dois deles oncogênicos (tipos 16 e 18), que respondem pelos casos de câncer de útero e os tipos 6 e 11, responsáveis por 90% das verrugas genitais.A da Glaxo também protege contra quatro tipos de HPV (16,18, 31 e 45), todos oncogênicos. “A proteção contra os tipos 16, 18 e 45 ficou próxima de 100%. Contra o 31, acima de 50%”, diz Edimilson Migowski, professor de Infectologia Pediátrica da Universidade Federal do Rio de Janeiro.Nos dois produtos, a aplicação é em três doses. A segunda aplicação da vacina da Merck é dada dois meses após a primeira e a terceira, quatro meses depois da segunda. Com a da Glaxo, a última dose deverá ser aplicada um mês após a segunda.O tempo de imunização das duas é de cerca de cinco anos, mas isso não significa que depois desse período a mulher terá de ser imunizada de novo - o prazo de cinco anos equivale ao tempo comprovado em pesquisas.Na Europa e nos Estados Unidos mais de 1 milhão de doses da Gardasil foram vendidas desde julho de 2006 - o preço da dose nos EUA é de US$ 120.Por aqui, o aval da Anvisa foi dado em agosto do ano passado. Desde então, pacientes, clínicas e hospitais já fazem encomendas junto a importadoras.Para 2006, o Instituto Nacional de Câncer (Inca) esperava 20 mil novos casos de câncer de colo de útero no Brasil. A letalidade da doença fica em torno de 50%. A contaminação do HPV pode ocorrer em qualquer tipo de contato com a área genital, mesmo oral ou por manuseio - os homens atuam como vetores da doença. Na maioria das vezes a infecção não tem sintomas. O HPV pode ser detectado pelo exame ginecológico papanicolau
A volta dos não foram embora: Cox-2 ou The Empire strikes back.
Confesso o meu cansaço em discutir "reposição hormonal" e, também "cox-2". Gostaria de falar sobre antihipertensivos, mas a Big Pharma não permite. Abaixo, texto do The Wall Street Journal sobre a volta dos que não foram. Destaco o seguinte parágrafo:
Novartis began quietly selling Prexige in 2005 while the other Cox-2s battled controversy. It launched the drug first in Brazil and later in the United Kingdom and Australia. Last year, Novartis received regulatory approval to begin selling Prexige across Europe. The drug's global sales last year climbed to $47 million from $8 million in 2005, Novartis said in its year-end financial results.
Drug Makers TryTo Bring BackCox-2 Inhibitors
By JEANNE WHALENJanuary 19, 2007; Page B1
Two years after Merck & Co. was forced to pull the painkiller Vioxx from the global market because of cardiovascular side effects, pain pills in the same class -- known as Cox-2 inhibitors -- are quietly attempting a comeback.
Drug giant Novartis AG yesterday said that it will start selling its Cox-2 inhibitor Prexige across Europe in coming months, and that it has completed the clinical trials necessary to resubmit the drug for U.S. Food and Drug Administration review this year. Thomas Ebeling, chief executive of Novartis pharmaceuticals, said the company hopes to begin selling Prexige in the U.S. by the first half of 2008, adding it believes Prexige can eventually attain global sales of $500 million to $1 billion a year.
That followed Merck's statement in November that it plans to seek FDA approval for Arcoxia, a Cox-2 inhibitor that the company already sells in Europe and other countries outside the U.S. Last August, Merck released preliminary data that suggested Arcoxia doesn't carry more cardiovascular risks than diclofenac, an older pain drug. However, some doctors said the study's data was limited and questioned the comparison of Arcoxia to diclofenac, which they say acts like a Cox-2 inhibitor in the first place -- rather than comparing it to a less-similar painkiller such as naproxen, sold under the brandname Aleve.
Cox-2 inhibitors were designed to be easier on the stomach than older pain pills such as ibuprofen and naproxen, which are associated with gastric bleeding in some patients. But several of the Cox-2 drugs became mired in safety scandals. Merck withdrew Vioxx from the global market in the fall of 2004 after a study showed it elevated patients' risk of heart attacks and strokes. In April 2005, Pfizer Inc. pulled its Bextra pain reliever from most markets world-wide amid FDA concerns about the drug's safety. Merck and Pfizer are still fighting big lawsuits from patients who took the pills. In the U.S., Pfizer's Celebrex is the only Cox-2 remaining on the market.
Some experts think the new Cox-2 drugs face a high regulatory hurdle in the U.S., given the legacy of their predecessors. "Things have changed dramatically in terms of FDA oversight, in part because of Vioxx and Bextra," says James Kirkpatrick, assistant professor of medicine in the University of Pennsylvania's cardiovascular division. "It's certainly a risky move to invest a lot of effort into it."
If the drugs don't get approval to be sold in the U.S., they will face an uncertain fate. Cox-2 drugs have sold in only small amounts in Europe, where governments have steered patients to cheaper, older drugs such as diclofenac and naproxen.
But some big drug makers believe there is still a market for Cox-2s that can prove themselves safe and effective -- particularly for pain sufferers with gastrointestinal problems.
"If you believe in one of your products, you have to be resilient," Novartis chief executive Daniel Vasella said in an interview.
Novartis spoke about its plans for Prexige as it reported that fourth-quarter net profit rose 23% to $1.66 billion from $1.35 billion in the year-earlier quarter, driven by strong sales of blood-pressure pill Diovan and cancer drug Gleevec. Sales increased 16% to $10.05 billion from $8.66 billion.
Novartis began quietly selling Prexige in 2005 while the other Cox-2s battled controversy. It launched the drug first in Brazil and later in the United Kingdom and Australia. Last year, Novartis received regulatory approval to begin selling Prexige across Europe. The drug's global sales last year climbed to $47 million from $8 million in 2005, Novartis said in its year-end financial results.
Novartis submitted Prexige for U.S. approval in 2002, but the FDA asked for additional safety and efficacy studies. In particular, the FDA was concerned that some patients taking a 400-milligram dose of Prexige showed signs of liver toxicity. The agency asked Novartis, based in Basel, Switzerland, to study the drug at a lower, 100-milligram dose. It was also concerned that Novartis hadn't studied the drug in enough overweight people and asked for an additional clinical trial in overweight Americans with osteoarthritis of the hip.
Novartis has now completed those studies and plans to submit them in the coming months to the FDA, which is likely to give the drug a tough review. To simplify its application, Novartis will for now ask the agency to approve Prexige for osteoarthritis only and not for acute pain, a second indication that it originally hoped to gain, said James Shannon, head of pharmaceutical development at Novartis.
Pfizer has made a major effort to reintroduce Celebrex to doctors and to revive direct-to-consumer advertising for the drug, causing sales to rebound after a steep fall in 2005. In the third quarter of last year, global sales reached $537 million, up 20% from the year-earlier quarter. Pfizer said at the time it expects 2006 sales to hit $2 billion, up from $1.7 billion the year before. Nonetheless, those numbers are significantly down from Celebrex's $3.3 billion in 2004 sales.
Merck said it will also seek a narrower FDA approval for Arcoxia than it had previously sought. Merck plans to ask for permission to sell Arcoxia to treat symptoms of osteoarthritis. Previously, it had sought approval for a range of uses, including treatment of rheumatoid arthritis.
By JEANNE WHALENJanuary 19, 2007; Page B1
Two years after Merck & Co. was forced to pull the painkiller Vioxx from the global market because of cardiovascular side effects, pain pills in the same class -- known as Cox-2 inhibitors -- are quietly attempting a comeback.
Drug giant Novartis AG yesterday said that it will start selling its Cox-2 inhibitor Prexige across Europe in coming months, and that it has completed the clinical trials necessary to resubmit the drug for U.S. Food and Drug Administration review this year. Thomas Ebeling, chief executive of Novartis pharmaceuticals, said the company hopes to begin selling Prexige in the U.S. by the first half of 2008, adding it believes Prexige can eventually attain global sales of $500 million to $1 billion a year.
That followed Merck's statement in November that it plans to seek FDA approval for Arcoxia, a Cox-2 inhibitor that the company already sells in Europe and other countries outside the U.S. Last August, Merck released preliminary data that suggested Arcoxia doesn't carry more cardiovascular risks than diclofenac, an older pain drug. However, some doctors said the study's data was limited and questioned the comparison of Arcoxia to diclofenac, which they say acts like a Cox-2 inhibitor in the first place -- rather than comparing it to a less-similar painkiller such as naproxen, sold under the brandname Aleve.
Cox-2 inhibitors were designed to be easier on the stomach than older pain pills such as ibuprofen and naproxen, which are associated with gastric bleeding in some patients. But several of the Cox-2 drugs became mired in safety scandals. Merck withdrew Vioxx from the global market in the fall of 2004 after a study showed it elevated patients' risk of heart attacks and strokes. In April 2005, Pfizer Inc. pulled its Bextra pain reliever from most markets world-wide amid FDA concerns about the drug's safety. Merck and Pfizer are still fighting big lawsuits from patients who took the pills. In the U.S., Pfizer's Celebrex is the only Cox-2 remaining on the market.
Some experts think the new Cox-2 drugs face a high regulatory hurdle in the U.S., given the legacy of their predecessors. "Things have changed dramatically in terms of FDA oversight, in part because of Vioxx and Bextra," says James Kirkpatrick, assistant professor of medicine in the University of Pennsylvania's cardiovascular division. "It's certainly a risky move to invest a lot of effort into it."
If the drugs don't get approval to be sold in the U.S., they will face an uncertain fate. Cox-2 drugs have sold in only small amounts in Europe, where governments have steered patients to cheaper, older drugs such as diclofenac and naproxen.
But some big drug makers believe there is still a market for Cox-2s that can prove themselves safe and effective -- particularly for pain sufferers with gastrointestinal problems.
"If you believe in one of your products, you have to be resilient," Novartis chief executive Daniel Vasella said in an interview.
Novartis spoke about its plans for Prexige as it reported that fourth-quarter net profit rose 23% to $1.66 billion from $1.35 billion in the year-earlier quarter, driven by strong sales of blood-pressure pill Diovan and cancer drug Gleevec. Sales increased 16% to $10.05 billion from $8.66 billion.
Novartis began quietly selling Prexige in 2005 while the other Cox-2s battled controversy. It launched the drug first in Brazil and later in the United Kingdom and Australia. Last year, Novartis received regulatory approval to begin selling Prexige across Europe. The drug's global sales last year climbed to $47 million from $8 million in 2005, Novartis said in its year-end financial results.
Novartis submitted Prexige for U.S. approval in 2002, but the FDA asked for additional safety and efficacy studies. In particular, the FDA was concerned that some patients taking a 400-milligram dose of Prexige showed signs of liver toxicity. The agency asked Novartis, based in Basel, Switzerland, to study the drug at a lower, 100-milligram dose. It was also concerned that Novartis hadn't studied the drug in enough overweight people and asked for an additional clinical trial in overweight Americans with osteoarthritis of the hip.
Novartis has now completed those studies and plans to submit them in the coming months to the FDA, which is likely to give the drug a tough review. To simplify its application, Novartis will for now ask the agency to approve Prexige for osteoarthritis only and not for acute pain, a second indication that it originally hoped to gain, said James Shannon, head of pharmaceutical development at Novartis.
Pfizer has made a major effort to reintroduce Celebrex to doctors and to revive direct-to-consumer advertising for the drug, causing sales to rebound after a steep fall in 2005. In the third quarter of last year, global sales reached $537 million, up 20% from the year-earlier quarter. Pfizer said at the time it expects 2006 sales to hit $2 billion, up from $1.7 billion the year before. Nonetheless, those numbers are significantly down from Celebrex's $3.3 billion in 2004 sales.
Merck said it will also seek a narrower FDA approval for Arcoxia than it had previously sought. Merck plans to ask for permission to sell Arcoxia to treat symptoms of osteoarthritis. Previously, it had sought approval for a range of uses, including treatment of rheumatoid arthritis.
Um gráfico interessante...
 Adivinhe que fenômeno representa o gráfico ao lado? Se, você respondeu o número de americanos com seguro-saúde garantido pelo empregador, acertou. O pico foi de 65% em 2000. Esses dados foram divulgados na edição de hoje do The Wall Street Journal. Ontem, o governador da Pennsilvania lançou o projeto de cobertura ampla aos moradores do Estado. Isso depois de Massachusetts, Vermont, Maine e California. Abaixo, trecho da reportagem do WSJ. Note que Mitt Rommey, ex-governador de Massachusetts é candidatíssimo a Presidente pelos republicanos. Há grande chance da cobertura universal ser a grande questão das próximas eleições, se a retirada do Iraque já tiver sido efetivada, obviamente.
Adivinhe que fenômeno representa o gráfico ao lado? Se, você respondeu o número de americanos com seguro-saúde garantido pelo empregador, acertou. O pico foi de 65% em 2000. Esses dados foram divulgados na edição de hoje do The Wall Street Journal. Ontem, o governador da Pennsilvania lançou o projeto de cobertura ampla aos moradores do Estado. Isso depois de Massachusetts, Vermont, Maine e California. Abaixo, trecho da reportagem do WSJ. Note que Mitt Rommey, ex-governador de Massachusetts é candidatíssimo a Presidente pelos republicanos. Há grande chance da cobertura universal ser a grande questão das próximas eleições, se a retirada do Iraque já tiver sido efetivada, obviamente.Then there's raw partisan politics. Democrats believe they have the political wind at their backs and that one reason they do is public anxiety about health care. Republicans feel a need to respond, and often look for approaches that rely less on government and more on market forces.
At the same time, some proponents of free trade seek ways to ease workers' anxieties about globalization so they might be less hostile to it. Recent initiatives at the state level reflect the public pressure for some kind of governmental action -- and at the same time increase the pressure for some federal action.
"Legislators and governors are feeling pressure from small businesses," says Katherine Swartz, a professor at the Harvard School of Public Health. She has another idea for dealing with the problem: government-subsidized reinsurance pools that might make it more affordable for employers to offer insurance.
Ms. Swartz says that "nobody believes anything is going to come out of Washington. States are more willing to say let's try something." Flush state budgets help. A survey by the Kaiser Family Foundation found that one-third of states, 17 in all, increased access to health coverage in 2006, often to low-income children. F
At the same time, some proponents of free trade seek ways to ease workers' anxieties about globalization so they might be less hostile to it. Recent initiatives at the state level reflect the public pressure for some kind of governmental action -- and at the same time increase the pressure for some federal action.
"Legislators and governors are feeling pressure from small businesses," says Katherine Swartz, a professor at the Harvard School of Public Health. She has another idea for dealing with the problem: government-subsidized reinsurance pools that might make it more affordable for employers to offer insurance.
Ms. Swartz says that "nobody believes anything is going to come out of Washington. States are more willing to say let's try something." Flush state budgets help. A survey by the Kaiser Family Foundation found that one-third of states, 17 in all, increased access to health coverage in 2006, often to low-income children. F
quinta-feira, 18 de janeiro de 2007
Saneamento venceu a enquete do BMJ
O British Medical Journal fez enquete para verificar qual foi o avanço médico-sanitário mais importante desde 1840. Ganhou Saneamento e Controle de Resíduos no voto de mais 11 mil respondentes. Abaixo o resultado final
Proportion (%)
Anaesthesia (13.9); Antibiotics (14.5); Chlorpromazine (0.6); Computers
(3.6); Discovery of DNA structure (8.8); Evidence-based medicine (5.6); Germ theory (7.4); Immunology (1.6); Medical imaging (x-rays, etc.)
(4.2); Oral contraceptive pill (7.4); Oral rehydration therapy (2.7); Risks of smoking (1.6); Sanitation (clean water and sewage disposal) (15.8); Tissue culture (0.4); Vaccines (11.8).
Anaesthesia (13.9); Antibiotics (14.5); Chlorpromazine (0.6); Computers
(3.6); Discovery of DNA structure (8.8); Evidence-based medicine (5.6); Germ theory (7.4); Immunology (1.6); Medical imaging (x-rays, etc.)
(4.2); Oral contraceptive pill (7.4); Oral rehydration therapy (2.7); Risks of smoking (1.6); Sanitation (clean water and sewage disposal) (15.8); Tissue culture (0.4); Vaccines (11.8).
0800: a era do anonimato chegou
Defendi no domingo, que não se discutisse como construir um metrô, mas sim, como auxiliar e apoiar quem sofre com uma tragédia, seja na terra, no ar ou na água. Ontem, Elio Gaspari na Folha de S.Paulo sem escrever uma vírgula sobre engenharia civil atingiu em cheio, o descaso das empreiteiras no acompanhamento dos familiares das vítimas e, nos vizinhos da obra.
Recentemente, mudei de casa e, obrigado a alterar serviços e endereços. Em curto espaço de tempo, tive contato com o maravilhoso mundo “0800”, onde tudo funciona, se funcionar, caso contrário, o consumidor está perdido, sem qualquer chance de ser atendido. Nada se comparou ao atendimento da TV a cabo, um verdadeiro desastre. Os operadores mentem na cara dura, ninguém é responsável. Ninguém conhece quem o atendeu anteriormente. Ângela?, desconheço! João Roberto, não sei de quem se trata!
Recentemente, mudei de casa e, obrigado a alterar serviços e endereços. Em curto espaço de tempo, tive contato com o maravilhoso mundo “0800”, onde tudo funciona, se funcionar, caso contrário, o consumidor está perdido, sem qualquer chance de ser atendido. Nada se comparou ao atendimento da TV a cabo, um verdadeiro desastre. Os operadores mentem na cara dura, ninguém é responsável. Ninguém conhece quem o atendeu anteriormente. Ângela?, desconheço! João Roberto, não sei de quem se trata!
Uma empresa de celular conseguiu a proeza de avisar no 0800 que deveria acessar a Internet para mudar o endereço, mas o próprio site informava que mudança de endereço somente pelo telefone! Estive também no fatídico 5 de dezembro tentando voar para Brasília. Antes do tumulto geral em Congonhas, na loja da TAM não havia um supervisor ou diretor tentado conter a confusão que se aproximava. Um descaso total e, principalmente: não há responsável! Salvaram-se integralmente: American Express e Conselho Regional de Medicina.
Vivemos a era "0800", a anomia total, ninguém é responsável por nada, ao contrário do praticado pelo mítico Comandante Rolim, da TAM>O Comandante Rolim morreu e, não deixou herdeiros. Há mais de dez anos viajei em companhia de um "esquerdista empertigado" que ao ver o Comandante na porta da aeronave saudando os passageiros e, depois deixando um impresso para comunicação de problemas cedeu: acho que o capitalismo tem salvação.
A doença de Fidel e o exercício profissional no Brasil
Um amigo que não quis enviar mensagem no blog informa que o acontecido com Fidel Castro não passou despercebido no setor governista ideologicamente "cubanista e fidelista" que "força a barra" para aprovar o projeto de "Reconhecimento Recíproco de Diplomas com Cuba". Tudo isso dentro da mística da qualidade superior da medicina cubana.
A melhor síntese desse problema foi a elaborada por Edson de Oliveira Andrade, presidente do Conselho Federal de Medicina no texto "O mito da medicina cubana" que pode ser acessado no http://www.portalmedico.org.br
Minha opinião é simples e, independente de qualquer juízo de valor sobre a qualidade da medicina em qualquer lugar do planeta: todos os formados - brasileiros ou não - fora do país que queiram exercer a medicina devem ser submetidos a prova de revalidação de diploma. Ponto final.
terça-feira, 16 de janeiro de 2007
A doença de Fidel e dos governantes.
O jornal El País noticiou que Fidel Castro teve uma diverticulite aguda com peritonite e, depois foi reoperado duas vezes pelas complicações (dizem da cirurgia primeira, mas eu acho que foi da própria doença). Informam que a equipe que operou "caiu em desgraça". Difícil comentar ou opinar sobre um caso clínico quando o médico tem todas as informações. Iimpossível, quando há somente um relato na imprensa. Tal como ocorre no apagão aéreo, enchentes em Minas e cratera do metro sempre há um "especialista" que "sabe tudo', mas "não fez nada".
No passado, o lendário Paulo Dudley-White, professor de cardiologia da Harvard, cuidou do então presidente e, também lenda americana, Eisenhower que sofrera um infarto do miocárdio ; O professor fez relatos técnicos que foram censurados na Inglaterra, afinal citava que o "presidente já está eliminando gases". George Pompidou estava tomando cortisona (literalmente estava na cara) e, negava doença. JK escondeu um infarto do miocárdio. Os casos Tancredo e Covas já foram muito discutidos.
Recentemente, tivemos a morte de Yasser Arafat sem relato da causa básica por imposição da mulher.
Qual a posição a ser tomada em caso de doenças de dirigentes políticos ou mesmo as "celebridades"? Minha opinião:
(1) apesar do problema que representa para a comunidade o seu afastamento, a decisão de noticiar deve ser do paciente ou de sua família.
(2) o relato deve ser técnico, sintético por meio de boletins médicos assinados pelo diretor da instituição, sem entrevista coletiva ou mesmo o "off".
(3) principal:médicos que estão envolvidos devem ficar quietos porque em qualquer manifestação, eles ferem o sigilo profissional. Os demais mais quietos ainda para não falarem besteira e, por causa de quinze segundos de glória, mostrarem-se ridículos ao comentar o que não conhecem. Inevitável, que o mesmo jornalista que entende de aviação, obras de grande porte também opinará sobre a doença e conduta.
segunda-feira, 15 de janeiro de 2007
Um festival de dados: a PNAD 2003
Abaixo a introdução de Francisco Viacava para essa edição da revista.
O Brasil é, possivelmente, um dos países que mais tem investido em informações na área da saúde. Entretanto, o crescente volume de dados armazenados em diversos sistemas desenvolvidos não tem sido totalmente aproveitado quando se trata da avaliação sistemática das políticas de saúde. Por outro lado, bases de dados de estatísticas vitais e de sistemas administrativos não contêm um conjunto importante de informações necessárias para a avaliação das políticas de saúde.
Dados de base populacional possibilitam a realização de análises para o conjunto da população tanto no que se refere às necessidades de saúde, quanto ao acesso e ao uso que têm sido feitos dos serviços de saúde. Permitem também, a avaliação da fonte de financiamento do uso de serviços de saúde pública e privada seja por plano de saúde ou pelo próprio bolso e a caracterização da população vinculada a cada uma dessas fontes.
Entretanto, no Brasil, os inquéritos de base populacional têm sido feitos de forma pontual, sem uma periodicidade definida e sem uma articulação mais estreita com os formuladores da política de saúde. O Ministério da Saúde tem investido recursos na realização desses inquéritos, mas não há uma política claramente definida a respeito de que dimensões deveriam ser periodicamente avaliadas. Com a realização da PNAD/2003, cujo questionário é basicamente o mesmo de 1998, foi possível ter pela primeira vez um acompanhamento da evolução de aspectos particulares dos planos de saúde, do acesso e da utilização de serviços que vêm sendo feitos pela população brasileira.
Dados de base populacional possibilitam a realização de análises para o conjunto da população tanto no que se refere às necessidades de saúde, quanto ao acesso e ao uso que têm sido feitos dos serviços de saúde. Permitem também, a avaliação da fonte de financiamento do uso de serviços de saúde pública e privada seja por plano de saúde ou pelo próprio bolso e a caracterização da população vinculada a cada uma dessas fontes.
Entretanto, no Brasil, os inquéritos de base populacional têm sido feitos de forma pontual, sem uma periodicidade definida e sem uma articulação mais estreita com os formuladores da política de saúde. O Ministério da Saúde tem investido recursos na realização desses inquéritos, mas não há uma política claramente definida a respeito de que dimensões deveriam ser periodicamente avaliadas. Com a realização da PNAD/2003, cujo questionário é basicamente o mesmo de 1998, foi possível ter pela primeira vez um acompanhamento da evolução de aspectos particulares dos planos de saúde, do acesso e da utilização de serviços que vêm sendo feitos pela população brasileira.
Falar é prata, calar é ouro.
Incrível, a leitura dos jornais e sites nesse fim de semana. Descobri que os jornalistas que entendiam de sistema penitenciário, de aviação (falavam de transponder, como se fosse um instrumento ordinário como um copo de água), também são experts em enchentes (e, de qualidade de lama), construção de metrô e salvamento de desabamento. Falar é prata, mas calar é ouro, diziam nossos avós. Solidariedade às famílias envolvidas e aos trabalhadores do resgate em Minas Gerais e São Paulo seria a melhor forma de manifestação.
domingo, 14 de janeiro de 2007
A reprodução assistida: crítica feroz do diretor do HC.
Jorge Hallak é médico urologista formado na FMUSP e, dirige o Centro de Reprodução Humana do HCFMUSP. Em O Estado de S.Paulo (14/01/07), Aureliano Biancarelli e Mônica Manir publicam entrevista com ele. O conteúdo é explosivo pelo grau de denúncia. Nada novo para quem conhece o que se passa nesse setor, movido a muito dinheiro, assessorias de imprensa eficazes que informam somente sucessos e, atitudes médicas discutíveis. Recomenda-se guardar a entrevista para apresentar a casais que procuram clínicas de infertilidade. A citação dos custos com berçário de alto risco merece ser destacada. Em suma, uma atitude corajosa, contra o corporativismo e, a favor da medicina.
Com a a palavra, o CREMESP e CFM. Abaixo a abertura da entrevista.
A espera e a chegada de um filho estão entre os eventos mais marcantes na vida do homem e da mulher. Significa a continuidade e a preservação da vida. Para cerca de 15% dos casais, número estimado de pares inférteis, só haverá espera, não chegada. O que seria uma festa, passa a ser uma seqüência de frustrações.Esse cenário está mudando. A tecnologia reprodutiva vem revertendo a lei da seleção natural, permitindo que quase a metade dos casais inférteis tenha filhos. Por conta dos altos custos, os procedimentos ainda estão restritos a uma minúscula parcela dos casais que necessitam, menos de 5%, no Brasil.Para muitos daqueles que podem pagar - cerca de R$ 40 mil por criança nascida viva -, a busca pela reprodução assistida tem sido uma peregrinação cheia de falsas promessas, procedimentos inúteis e gastos desnecessários.'Se os casais que procuram pela reprodução assistida fossem bem informados, um quarto deles deixaria de fazê-la', diz o urologista Jorge Hallak, diretor científico do Centro de Reprodução da Universidade de São Paulo, 41 anos, sem filhos.Embora o Brasil esteja na ponta da tecnologia reprodutiva, Hallak diz que a prática sem regras nem ética de muitas clínicas vem transformando o Brasil num país de 'turismo reprodutivo', atraindo especialmente casais europeus.Procedimentos como a redução embrionária, a implantação de muitos embriões, a escolha do sexo do bebê e intervenções sem diagnóstico são praticados sem nenhuma fiscalização. 'O Brasil se transformou numa fábrica de bebê de proveta, onde não há tempo para se fazer o diagnóstico. Aqui, de cada 100 casais que chegam para fazer inseminação artificial, 95% acabam fazendo, mas menos da metade deles precisariam.'O emprego do ICSI - técnica sofisticada que permite a implantação de um único espermatozóide dentro do óvulo - 'deve ser o último recurso para um casal que não consegue ter filhos', diz o médico. 'No Brasil, é sempre a primeira opção, porque é mais rápida e dá mais dinheiro. A reprodução assistida virou uma terra de ninguém.'As clínicas 'só não informam aos pais angustiados de que esses recursos provocam gravidez múltipla, parto prematuro, nascimento de baixo peso e risco de morte para a mãe e o bebê'.Fascinante, a tecnologia reprodutiva coloca questões éticas ainda não estabelecidas, como os bancos de sêmen, o congelamento de embriões, o consentimento informado dos pais, a barriga de aluguel, a escolha de embriões com base na evidência de doenças e, em última instância, o direito do embrião à vida. Essas são algumas das questões a serem tratadas no Núcleo de Pensamento Jurídico em Reprodução Humana, instalado na semana passada por profissionais da USP e do Mackenzie, e do qual Jorge Hallak é o coordenador técnico científico. Abaixo, trechos de sua entrevista
sexta-feira, 12 de janeiro de 2007
Deu no JN: bloqueio do sarampo na Bahia.
Na fase ufanística que se encontra esse blog, não dá para negar registro à reportagem do Jornal Nacional (12/01/07) mostrando a ação da Secretaria de Estado da Saúde da Bahia em fazer o bloqueio dos casos de sarampo descritos no sertão baiano. Talvez, trazido por um europeu que visitou o garimpo. Um trabalho sanitário que mostra a organização de vários serviços de saúde pública no país. O SUS representou um avanço relativamente menor no Rio de Janeiro e em São Paulo comparado aos demais estados (e, mesmo no interior do Estado do Rio), por isso haja um certo desconforto em aceitar a melhoria que o SUS representa.
Fundação Gates: dúvidas, muitas dúvidas.
Reproduzo abaixo, o post de Paulo Moreira Leite, (http://www.estadao.com.br/blogs/paulo) que discorre sobre a fundação Bill Gates a partir de reportagem do Los Angeles Times. Já critiquei aqui a questão da "vacina do amarelão" que estimulada pela Fundação Melinda e Bill Gates estará sendo produzida no Brasil (Instituto Butantan) para ser aplicado em algumas áreas do país. As prioridades do país devem ser estabelecidas no próprio país a partir da realidade concreta. Minas Gerais está com taxas ascendentes de homicídio na região metropolitana, o Jeca Tatu (se existir) com amarelão, coitado, morre de anemia, mas por sangramento devido a arma de fogo.
Bill Gates e o mito do capitalista bondoso
Fiquei impressionado com uma reportagem do Los Angeles Times sobre a Fundação de Bill Gates, a mais rica do planeta, com orçamento de U$ 70 bilhões. Você e eu já lemos muitas reportagens sobre as filantropias dessa fundação. A reportagem do Los Angeles Times (reproduzida pelo Estado) mostra que a Fundação "doa pelo menos 5% de seu orçamento todos os anos, e com isso evita pagar a maioria dos impostos." Já os 95% restantes são investidos "em companhias ranqueadas entre as piores poluidoras americanas, companhias farmacêuticas que estabelecem preços para remédios fora do alcance de pacientes" e assim por diante. Conforme o jornal, investimentos que totalizam pelo menos U$ 8,7 bilhões foram feitos "em companhias que contrariam a filosofia de preocupação social da fundação."Esse tipo de descoberta não chega a ser novo. Boa parte das empresas brasileiras deixam de pagar impostos devidos para realizar investimentos de marketing -- com o nome de apoio cultural. Outras empresas investem uma migalha social -- para receber banquetes privados. Sou um otimista e acredito na espécie humana. Mas é bom desconfiar um pouco mais da humanidade depois que Milton Friedman lembrou que não existe almoço grátis.
Fiquei impressionado com uma reportagem do Los Angeles Times sobre a Fundação de Bill Gates, a mais rica do planeta, com orçamento de U$ 70 bilhões. Você e eu já lemos muitas reportagens sobre as filantropias dessa fundação. A reportagem do Los Angeles Times (reproduzida pelo Estado) mostra que a Fundação "doa pelo menos 5% de seu orçamento todos os anos, e com isso evita pagar a maioria dos impostos." Já os 95% restantes são investidos "em companhias ranqueadas entre as piores poluidoras americanas, companhias farmacêuticas que estabelecem preços para remédios fora do alcance de pacientes" e assim por diante. Conforme o jornal, investimentos que totalizam pelo menos U$ 8,7 bilhões foram feitos "em companhias que contrariam a filosofia de preocupação social da fundação."Esse tipo de descoberta não chega a ser novo. Boa parte das empresas brasileiras deixam de pagar impostos devidos para realizar investimentos de marketing -- com o nome de apoio cultural. Outras empresas investem uma migalha social -- para receber banquetes privados. Sou um otimista e acredito na espécie humana. Mas é bom desconfiar um pouco mais da humanidade depois que Milton Friedman lembrou que não existe almoço grátis.
China: cobrança em hospital público
Depois da demonstração da nossa "superioridade" sobre o sistema americano na distribuição de insulina, abaixo reproduzo um resumo de reportagem publicada hoje com acesso livre no The Lancet (http://thelancet.com) que relata os protestos violentes contra cobrança de atendimento em hospital público, isso na República Popular da China, tida como "socialista", "comunista".
If the Chinese mandarins now drawing up a major overhaul of their country's health-care system needed a reminder about the urgency of their task, it was provided in violent fashion at the end of last year with a hospital riot in the birthplace of Deng Xiaoping. About 2000 protesters went on the rampage in Guangan, Sichuan Province, in November after reports that a young boy was denied life-saving treatment because his guardians could not afford the £50 treatment fee. Although the facts of the case are disputed, it highlights the growing sense of public unease about a profit-orientated health-care system in which doctors are suspected of worrying more about payment than their patients' lives.
If the Chinese mandarins now drawing up a major overhaul of their country's health-care system needed a reminder about the urgency of their task, it was provided in violent fashion at the end of last year with a hospital riot in the birthplace of Deng Xiaoping. About 2000 protesters went on the rampage in Guangan, Sichuan Province, in November after reports that a young boy was denied life-saving treatment because his guardians could not afford the £50 treatment fee. Although the facts of the case are disputed, it highlights the growing sense of public unease about a profit-orientated health-care system in which doctors are suspected of worrying more about payment than their patients' lives.
quinta-feira, 11 de janeiro de 2007
Distribuição de Insulina: dilema nos EUA, realidade em São Paulo.
Hoje, nos Estados Unidos está em debate a distribuição de insulina a diabéticos conforme reportagem de The New York Times. Em São Paulo, nas unidades básicas de saúde, a insulina é distribuída gratuitamente desde.......1984! No Hospital das Clínicas desde.....1944?? Eu testemunhei momentos de discontinuidade tanto nas UBS como no HC, mas houve mais oferta de medicamento do que demanda. Acreditem. Hoje, tanto nas UBS (grátis) como nas "Farmácia Popular" (preço reduzido), há insulina para todos. Depois, falam mal do sistema público.
obs.1 não tenho idéia da distribuição fora de São Paulo, por isso omiti a informação.
obs2. Nos anos 70/80 havia a idéia de que produzir insulina era uma atividade estratégica para um país, daí a Biobras em Montes Claros, MG. Essa é outra história a ser recontada.
Bridling at Insulin’s Cost, States Push for Generics
Kate Medley for The New York Times
Rose McDaniels working at the Great Harvest Bread bakery in Jackson, Miss. She spends more than 10 percent of her income on insulin. The drug cost state Medicaid programs $500 million in 2005. And in the face of an epidemic of diabetes, the governors are asking why there is no cheaper generic version of a drug that, in one form or another, has been used since the 1920s. Rose McDaniels is wondering, too. She is a bakery worker in Mississippi, the state with the highest incidence of the disease. The $115 a month she spends on insulin consumes more than 10 percent of her income. She sometimes cuts back her dosage to make it from payday to payday, though she knows the risks. "I’m trying to make it until the next available dollar I can spare,” she said. The issue involves questions of politics and profits, as well as science.
Insulin is caught in a commercial tug of war between brand-name drug companies that want to protect their franchises and generic drug makers that want to produce their own insulin products. Recently, each side has stepped up efforts to advance its side of the argument, and there are signs that Congress is ready to make life easier for generic drug companies. People with diabetes in this country, as well as government and private insurers, spend a combined $3.3 billion a year on insulin. Analysts say the price of insulin might drop by 25 percent if generic versions became available.
Kate Medley for The New York Times
Rose McDaniels working at the Great Harvest Bread bakery in Jackson, Miss. She spends more than 10 percent of her income on insulin. The drug cost state Medicaid programs $500 million in 2005. And in the face of an epidemic of diabetes, the governors are asking why there is no cheaper generic version of a drug that, in one form or another, has been used since the 1920s. Rose McDaniels is wondering, too. She is a bakery worker in Mississippi, the state with the highest incidence of the disease. The $115 a month she spends on insulin consumes more than 10 percent of her income. She sometimes cuts back her dosage to make it from payday to payday, though she knows the risks. "I’m trying to make it until the next available dollar I can spare,” she said. The issue involves questions of politics and profits, as well as science.
Insulin is caught in a commercial tug of war between brand-name drug companies that want to protect their franchises and generic drug makers that want to produce their own insulin products. Recently, each side has stepped up efforts to advance its side of the argument, and there are signs that Congress is ready to make life easier for generic drug companies. People with diabetes in this country, as well as government and private insurers, spend a combined $3.3 billion a year on insulin. Analysts say the price of insulin might drop by 25 percent if generic versions became available.
Now, as the nation’s diabetes rate climbs, political pressure is mounting on the Food and Drug Administration to open the door to generic versions. And Gov. Haley Barbour of Mississippi, a Republican, and 10 other governors have asked the F.D.A. to ease the way for generic insulin.
“To have a lower-cost solution for our very large diabetic population is in the interest of the state and the interest of these people,” Governor Barbour said.
“To have a lower-cost solution for our very large diabetic population is in the interest of the state and the interest of these people,” Governor Barbour said.
In Congress, some members see the generic-insulin issue as having implications for the nation’s health care bill that go well beyond diabetes treatments. Insulin is one of the most widely used of a growing category of drugs known as biologics — medicines, made from living organisms, that are the fastest-growing segment of pharmaceutical spending. Many believe that if generic insulin is approved, that would open the door to development of generic versions of other, more complex biologics. Some include popular and expensive treatments for rheumatoid arthritis, cancer and other diseases that cost tens of thousands of dollars a year. The colorectal cancer drug Avastin, for example, costs $4,000 a month.
Some generic drug manufacturers say that a first step would be for the F.D.A. to adopt guidelines explaining the testing and documentation that would be required for the approval of insulin. The 11 governors from states with high Medicaid spending on insulin are pressing for such guidelines, and generic drug manufacturers say they need them to proceed with applications for insulin. But the issue has been clouded by a series of regulatory delays by the F.D.A. The agency announced in 2001 that it was developing guidelines for approving generic insulin and human growth hormone, another widely used and long available biologic.
Some generic drug manufacturers say that a first step would be for the F.D.A. to adopt guidelines explaining the testing and documentation that would be required for the approval of insulin. The 11 governors from states with high Medicaid spending on insulin are pressing for such guidelines, and generic drug manufacturers say they need them to proceed with applications for insulin. But the issue has been clouded by a series of regulatory delays by the F.D.A. The agency announced in 2001 that it was developing guidelines for approving generic insulin and human growth hormone, another widely used and long available biologic.
Those guidelines have never been released, however, partly, critics say, because of pressure from the Biotechnology Industry Organization, which represents the brand-name makers of biologic drugs.
The association argues that the same shortcuts to approval that are granted for conventional generic drugs are not applicable to biologics, even simple ones like insulin. Those shortcuts include an exemption from the long clinical testing required for approval of brand-name drugs. The trade group has been working to make sure generic drug companies cannot piggyback on the clinical studies done by drug innovators.
The association argues that the same shortcuts to approval that are granted for conventional generic drugs are not applicable to biologics, even simple ones like insulin. Those shortcuts include an exemption from the long clinical testing required for approval of brand-name drugs. The trade group has been working to make sure generic drug companies cannot piggyback on the clinical studies done by drug innovators.
The F.D.A. says that it is studying the issue, but believes that it is more appropriate to develop guidelines that would apply to all biologics rather than to issue guidelines for individual products like insulin and human growth hormone. The agency has said it will release a scientific background paper this spring that examines the issue. But Senator Orrin Hatch, a Utah Republican, and Representative Henry Waxman, a California Democrat, asked last year why the guidelines for insulin and human growth hormone had not been released. “There is simply no excuse — scientific, legal or otherwise — for the F.D.A. to delay the release of these guidelines,” the lawmakers said in a letter to the F.D.A.
As Congress looks for ways to reduce health care spending and broaden access to drugs, Mr. Waxman, who heads the House Oversight and Government Reform Committee, has said that legislation encouraging the development of generic biologics is one of his top priorities.
O crime não compensa, mesmo. Ex-detentos morrem muito mais.
Apesar da tradição ocidental em "glamourizar" (existe esse verbo?) o crime e criminosos, desde o "O Poderoso Chefão" de Mário Puzo até "O Guri" de Chico Buarque, ser mal não compensa. Além de vaga cativa no inferno, o caminho a ele é mais curto. Vejam, o resumo do artigo abaixo publicado no The New England Journal of Medicine sobre mortalidade de ex-detentos.
Release from Prison — A High Risk of Death for Former Inmates
Ingrid A. Binswanger, M.D., Marc F. Stern, M.D., Richard A. Deyo, M.D., Patrick J. Heagerty, Ph.D., Allen Cheadle, Ph.D., Joann G. Elmore, M.D., and Thomas D. Koepsell, M.D.
Background The U.S. population of former prison inmates is large and growing. The period immediately after release may be challenging for former inmates and may involve substantial health risks. We studied the risk of death among former inmates soon after their release from Washington State prisons.
Methods We conducted a retrospective cohort study of all inmates released from the Washington State Department of Corrections from July 1999 through December 2003. Prison records were linked to the National Death Index. Data for comparison with Washington State residents were obtained from the Wide-ranging OnLine Data for Epidemiologic Research system of the Centers for Disease Control and Prevention. Mortality rates among former inmates were compared with those among other state residents with the use of indirect standardization and adjustment for age, sex, and race.
Results Of 30,237 released inmates, 443 died during a mean follow-up period of 1.9 years. The overall mortality rate was 777 deaths per 100,000 person-years. The adjusted risk of death among former inmates was 3.5 times that among other state residents (95% confidence interval [CI], 3.2 to 3.8). During the first 2 weeks after release, the risk of death among former inmates was 12.7 (95% CI, 9.2 to 17.4) times that among other state residents, with a markedly elevated relative risk of death from drug overdose (129; 95% CI, 89 to 186). The leading causes of death among former inmates were drug overdose, cardiovascular disease, homicide, and suicide.
Conclusions Former prison inmates were at high risk for death after release from prison, particularly during the first 2 weeks. Interventions are necessary to reduce the risk of death after release from prison.
Ingrid A. Binswanger, M.D., Marc F. Stern, M.D., Richard A. Deyo, M.D., Patrick J. Heagerty, Ph.D., Allen Cheadle, Ph.D., Joann G. Elmore, M.D., and Thomas D. Koepsell, M.D.
Background The U.S. population of former prison inmates is large and growing. The period immediately after release may be challenging for former inmates and may involve substantial health risks. We studied the risk of death among former inmates soon after their release from Washington State prisons.
Methods We conducted a retrospective cohort study of all inmates released from the Washington State Department of Corrections from July 1999 through December 2003. Prison records were linked to the National Death Index. Data for comparison with Washington State residents were obtained from the Wide-ranging OnLine Data for Epidemiologic Research system of the Centers for Disease Control and Prevention. Mortality rates among former inmates were compared with those among other state residents with the use of indirect standardization and adjustment for age, sex, and race.
Results Of 30,237 released inmates, 443 died during a mean follow-up period of 1.9 years. The overall mortality rate was 777 deaths per 100,000 person-years. The adjusted risk of death among former inmates was 3.5 times that among other state residents (95% confidence interval [CI], 3.2 to 3.8). During the first 2 weeks after release, the risk of death among former inmates was 12.7 (95% CI, 9.2 to 17.4) times that among other state residents, with a markedly elevated relative risk of death from drug overdose (129; 95% CI, 89 to 186). The leading causes of death among former inmates were drug overdose, cardiovascular disease, homicide, and suicide.
Conclusions Former prison inmates were at high risk for death after release from prison, particularly during the first 2 weeks. Interventions are necessary to reduce the risk of death after release from prison.
Assinar:
Comentários (Atom)